Method for preparing main intermediate of dabigatran etexilate through enzymatic reaction
A technology of dabigatran etexilate and enzymatic reaction, applied in the field of chemical industry and chemical medicine, can solve the problems of low yield, cumbersome operation, affecting yield, etc., achieve strong selectivity, increase reaction yield, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
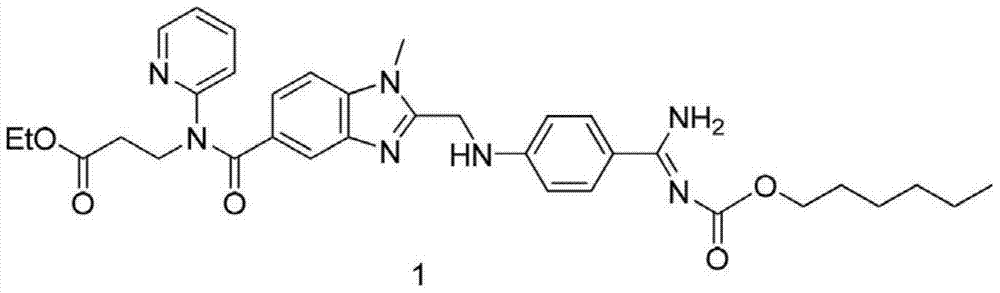
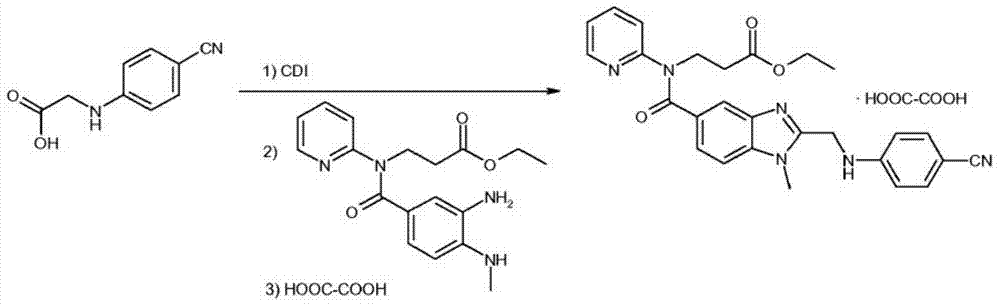
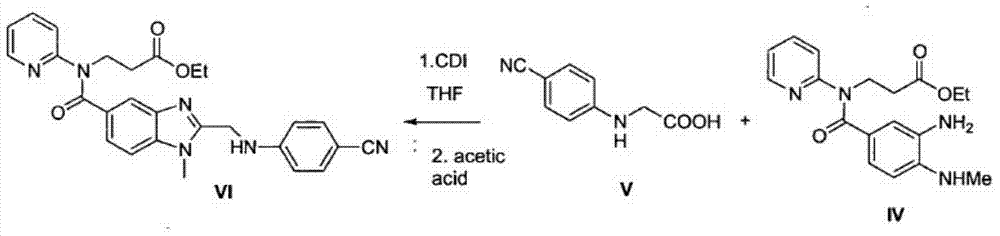
[0032] The intermediate 3-[4-methylamino-3-amino-N-(2-pyridyl)-benzamido]-acrylic acid ethyl ester 100g and the intermediate 2-(4-cyanoanilino)acetic acid 51.4 The molar ratio of g is 1:1. Add it into tetrahydrofuran to dissolve, add 30g of immobilized enzyme Novozym435 into the reaction solution, and stir at room temperature for 24h. Liquid phase detection raw material reaction is complete.
[0033] Remove the immobilized enzyme Novozym435 by filtration, distill off the solvent tetrahydrofuran, add 400ml of ethyl acetate and 200ml of purified water to wash, distill off the ethyl acetate, add the obtained oil to 200ml of glacial acetic acid and react at 110°C for 2 hours after the reaction is complete, distill off the acetic acid, add Wash with 400 ml of ethyl acetate and 200 ml of purified water, and evaporate the ethyl acetate to obtain the product. The yield was 75%, and the purity by liquid phase detection was 92%.
Embodiment 2
[0035] The intermediate 3-[4-methylamino-3-amino-N-(2-pyridyl)-benzamido]-acrylic acid ethyl ester 100g and the intermediate 2-(4-cyanoanilino)acetic acid 51.4 The molar ratio of g is 1:1. Add it into 400ml of dichloromethane to form a suspension, add 30g of immobilized enzyme Novozym435 into the reaction solution, and stir at room temperature for 24h. Liquid phase detection raw material reaction is complete.
[0036] Remove the immobilized enzyme Novozym435 by filtration, wash with 200ml of purified water, distill off the dichloromethane, add the obtained oil to 200ml of glacial acetic acid at 110°C for 2 hours, and after the reaction is complete, evaporate the acetic acid, add 400ml of ethyl acetate and wash with 200ml of purified water. Ethyl acetate was evaporated to obtain the product with a yield of 78% and a liquid phase detection purity of 94%.
Embodiment 3
[0038] The intermediate 3-[4-methylamino-3-amino-N-(2-pyridyl)-benzamido]-acrylic acid ethyl ester 100g and the intermediate 2-(4-cyanoanilino)acetic acid 51.4 The molar ratio of g is 1:1. Add it to 300ml of dichloromethane to form a suspension, add 100ml of ionic liquid [bmim]BF4 and add 30g of immobilized enzyme Novozym435 to the reaction solution, and stir at room temperature for 10h. Liquid phase detection raw material reaction is complete.
[0039] Remove the immobilized enzyme Novozym435 by filtration, evaporate the solvent dichloromethane, add 400ml of ethyl acetate to separate the organic layer, wash the organic layer with 200ml of purified water, evaporate the ethyl acetate, add the obtained oil to 200ml of glacial acetic acid and react at 110°C After 2 hours of reaction, evaporate the acetic acid, add 400ml of ethyl acetate and 200ml of purified water for washing, and evaporate the ethyl acetate to obtain the product. The yield was 81%, and the purity by liquid pha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com