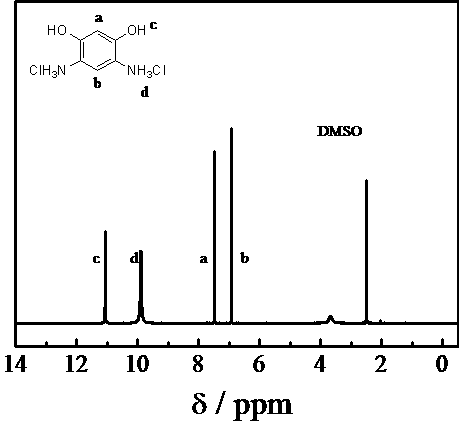
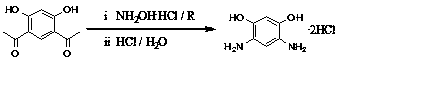
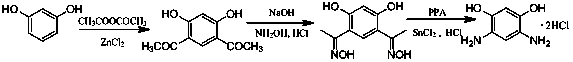Method for preparing 4,6-diaminoresorcinol dihydrochloride through one-pot synthesis
A technology of diaminoresorcinol hydrochloride and diacetylresorcinol is applied in the field of preparation of 4,6-diaminoresorcinol hydrochloride (DAR·2HCl), which can solve the problem of production efficiency It can improve the efficiency and yield, simplify the process flow, and promote the development of industrialization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] DAR hydrochloride was prepared by one-pot method at 65℃ and the molar ratio of hydroxylamine hydrochloride / polyphosphoric acid / resorcinol was 2.2 / 3 / 1.
[0020] Weigh polyphosphoric acid (PPA, 15.80g) and 4,6-diacetylresorcinol (3.00g) in a 100mL three-neck flask, add hydroxylamine hydrochloride (2.15g) to it under stirring conditions, and raise the temperature to 65°C, take samples every half hour for HPLC, observe the reaction, after the reaction is complete, cool to room temperature, add hydrochloric acid (6mol / L, 20 mL) and SnCl 2 (0.05g), continue to stir and raise the temperature to 100°C, and react for 2 hours. After cooling in air, the crystals were precipitated, filtered with suction, washed with hydrochloric acid, washed with ethanol, and vacuum-dried in a glass drying oven. The conversion rate was 43.5%.
Embodiment 2
[0022] DAR hydrochloride was prepared by one-pot method under the condition of 80℃ and the molar ratio of hydroxylamine hydrochloride / polyphosphoric acid / resorcinol at 2.2 / 3 / 1.
[0023] Weigh polyphosphoric acid (PPA, 15.80g) and acetyl resorcinol (3.00g) into a 100mL three-necked flask. Under stirring conditions, add hydroxylamine hydrochloride (2.15g) to it, raise the temperature to 80°C, take samples for HPLC every half hour, observe the reaction, after the reaction is completed, cool to room temperature, add hydrochloric acid (6mol / L, 20 mL ) and SnCl 2 (0.05g), continue to stir and raise the temperature to 100°C, and react for 2 hours. After cooling in air, crystals were precipitated, filtered with suction, washed with hydrochloric acid, washed with ethanol, and vacuum-dried in a glass drying oven. The conversion rate was 75.5%.
Embodiment 3
[0025] DAR hydrochloride was prepared by one-pot method at 95°C and the molar ratio of hydroxylamine hydrochloride / polyphosphoric acid / resorcinol was 2.2 / 3 / 1.
[0026] Weigh polyphosphoric acid (PPA, 15.80g) and acetyl resorcinol (3.00g) into a 100mL three-necked flask. Under stirring conditions, add hydroxylamine hydrochloride (2.15g) to it, raise the temperature to 95°C, take samples for HPLC every half hour, observe the reaction, after the reaction is completed, cool to room temperature, add hydrochloric acid (6mol / L, 20 mL ) and SnCl 2(0.05g), continue to stir and raise the temperature to 100°C, and react for 2 hours. After cooling in air, crystals were precipitated, filtered with suction, washed with hydrochloric acid, washed with ethanol, and vacuum-dried in a glass drying oven. The conversion rate was 69.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com