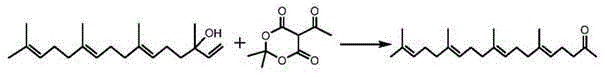
A kind of preparation method of teprenone
A technology of teprenone and acetylmelonic acid, which is applied in the field of drug synthesis technology, can solve the problems of being unable to determine whether it can be used in clinical practice, unable to directly use in actual production, and cumbersome post-processing process, so as to facilitate popularization and application , easy to control, simple method effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
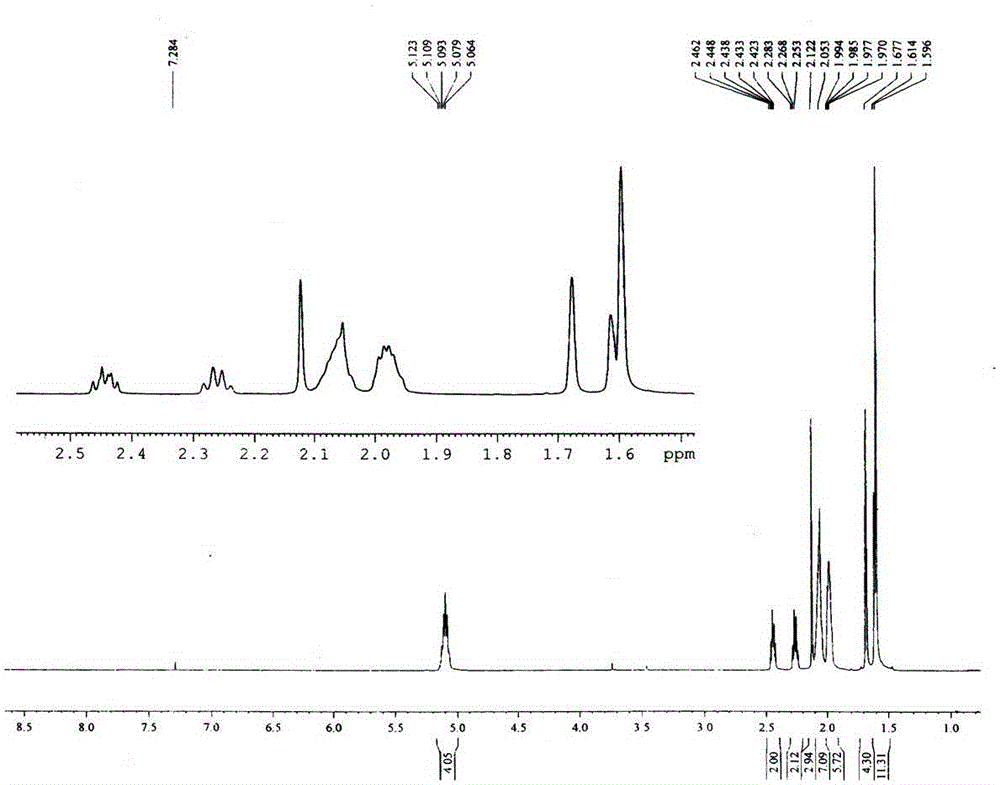
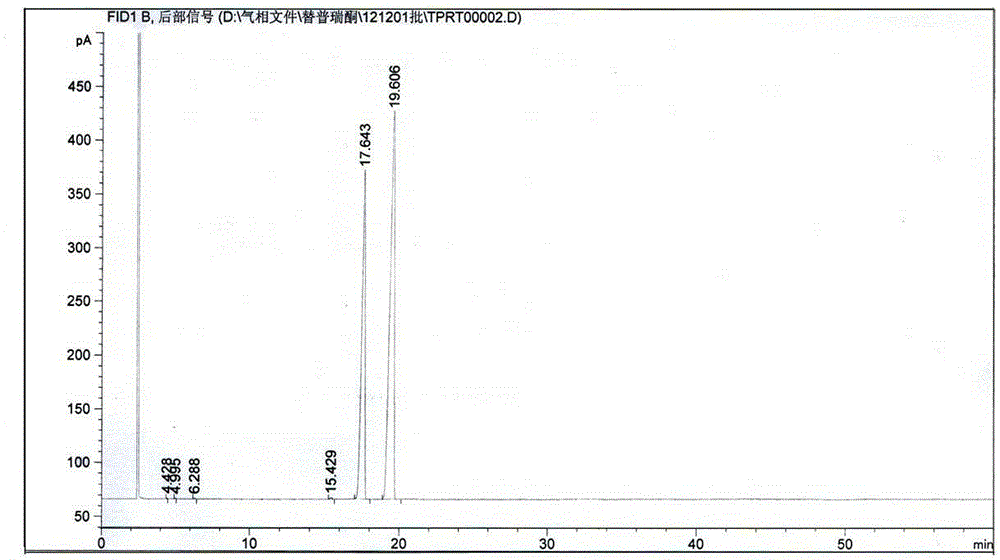
Image
Examples
Embodiment 1
[0026] Add 100 mL of geranyllinalool (0.3 mol), 68 g of acetylmelonic acid (0.36 mol) and 6.2 g of aluminum isopropoxide (0.03 mol) into the flask, and dissolve them in 600 mL of p-xylene. The flask was immersed in an oil bath preheated to 150°C and stirred for 8 hours. The reaction mixture was cooled to room temperature, concentrated with a rotary evaporator, and 50 mL of NaHCO with a concentration of 5% by mass 3 solution, washed with 300 mL of CH 2 Cl 2 Extract three times, combine the organic layers, and wash the organic phase with anhydrous MgSO 4 dry. Filtration, concentration, and molecular distillation of the concentrated solution, the feed rate is 2mL / min, the feed inlet temperature is 70°C, the heating temperature is 140°C, and the vacuum degree is 2.5Pa. The organic phase after molecular rectification is further reduced by a packed tower. Pressure distillation, the vacuum degree of the oil pump is 10Pa, glass filler, the rectification reflux ratio is gradually i...
Embodiment 2
[0028] Add 100 mL of geranyllinalo (0.3 mol), 85 g of acetylmelonic acid (0.45 mol) and 6.2 g of aluminum isopropoxide (0.03 mol) into the flask, and dissolve them in 200 mL of p-xylene. The flask was immersed in an oil bath preheated to 160°C and stirred for 6 hours. The reaction mixture was cooled to room temperature, concentrated with a rotary evaporator, and 50 mL of NaHCO with a concentration of 5% by mass 3 solution washed with 600 mL of CH 2 Cl 2 Extract three times, combine the organic layers, and wash the organic phase with anhydrous MgSO 4 dry. Filtration, concentration, and molecular distillation of the concentrated solution, the feed rate is 2mL / min, the feed inlet temperature is 60°C, the heating temperature is 160°C, and the vacuum degree is 10Pa. The organic phase after molecular rectification is further decompressed by a packed tower Distillation, the vacuum degree of the oil pump is 5Pa, glass packing, the rectification reflux ratio is gradually increased ...
Embodiment 3
[0030] Add 100 mL of geranyllinalool (0.3 mol), 85 g of acetylmelonic acid (0.45 mol) and 3.1 g of aluminum isopropoxide (0.015 mol) into the flask, and dissolve them in 400 mL of p-xylene. The flask was immersed in an oil bath preheated to 160°C and stirred for 10 hours. The reaction mixture was cooled to room temperature, concentrated with a rotary evaporator, and 50 mL of NaHCO with a concentration of 5% by mass 3 solution, washed with 300 mL of CH 2 Cl 2 Extract three times, combine the organic layers, and use anhydrous MgSO for the organic phase 4 dry. Filtration, concentration, and molecular distillation of the concentrated solution, the feed rate is 2mL / min, the feed inlet temperature is 60°C, the heating temperature is 140°C, and the vacuum degree is 2.5Pa. The organic phase after molecular rectification is further reduced by a packed tower. Pressure distillation, the vacuum degree of the oil pump is 8Pa, glass packing, the rectification reflux ratio is gradually i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com