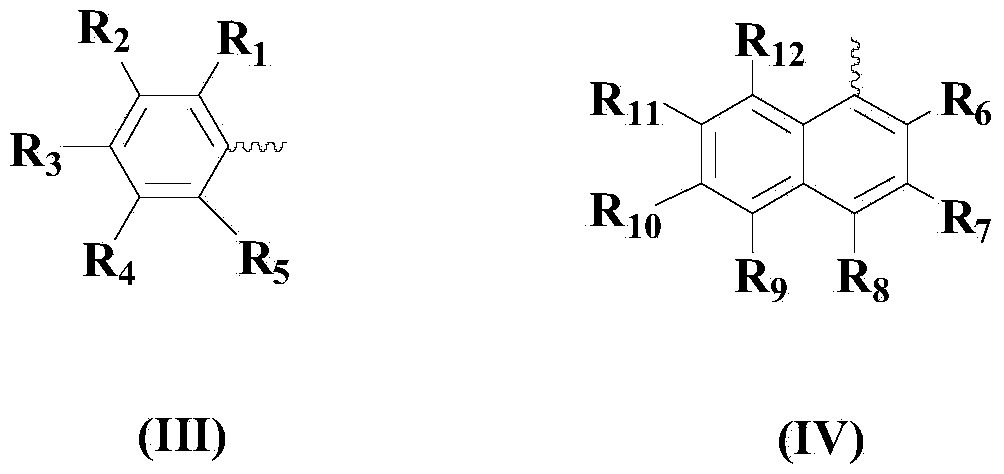
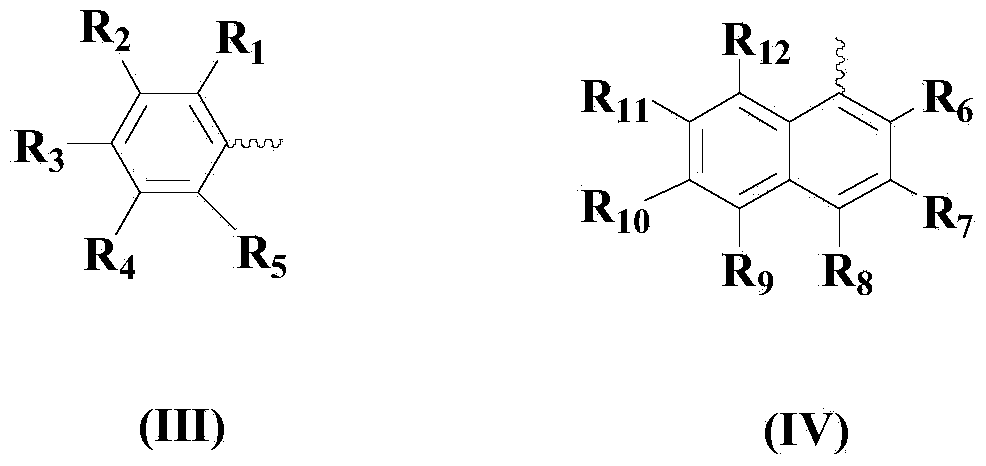
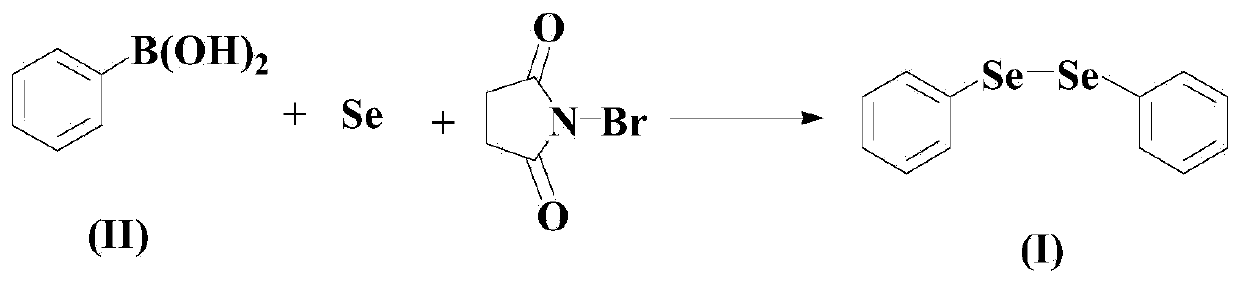
Diaryl diselenide compound synthesis method
A synthesis method and compound technology, applied in organic chemistry and other directions, can solve the problems of side reaction functional group compatibility, deterioration, and high reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the synthesis of diphenyl diselenide
[0049]
[0050] In a dry and clean reactor, add 50ml of solvent THF, then add 10mmol of compound (II), 10mmol of elemental selenium, 5mmol of N-bromosuccinimide and 15mmol of pyridine in sequence. The reaction was stirred for 16 hours.
[0051] After the reaction was finished, the reaction system was cooled to room temperature, and then the solvent was removed by rotary evaporation with a rotary evaporator to remove the solvent from the mixture obtained after the reaction, and the residue was purified by 300-400 mesh silica gel column chromatography to obtain the target product. The yield was It is 98.7%, and the purity is 99.8% (HPLC).
[0052] NMR: 1 H NMR (CDCl 3 ,500MHz):δ7.64-7.62(m,4H),7.26-7.29(m,6H);
[0053] 13 C NMR (CDCl 3 ,125MHz): δ131.5(2C), 130.9(4C), 129.2(4C), 127.7(2C).
Embodiment 2
[0054] Embodiment 2: the synthesis of two (p-methylphenyl) diselenides
[0055]
[0056] In a dry and clean reactor, add 50ml solvent 1,2-dichloroethane, then add 10mmol (II) compound, 12.5mmol elemental selenium, 10mmol N-bromosuccinimide and 20mmol pyridine successively, and react The system was stirred and reacted at 50° C. for 14 hours under an air atmosphere.
[0057] After the reaction was finished, the reaction system was cooled to room temperature, and then the solvent was removed by rotary evaporation with a rotary evaporator to remove the solvent from the mixture obtained after the reaction, and the residue was purified by 300-400 mesh silica gel column chromatography to obtain the target product. The yield was It is 97.6%, and the purity is 99.2% (HPLC).
[0058] NMR: 1 H NMR (CDCl 3 ,500MHz):δ7.49-7.53(m,4H),7.08-7.11(d,4H),2.37(s,6H);
[0059] 13 C NMR (CDCl 3 ,125MHz): δ137.9(2C), 132.3(4C), 129.9(4C), 127.7(2C), 21.1(2C).
Embodiment 3
[0060] Embodiment 3: the synthesis of two (p-methoxyphenyl) diselenides
[0061]
[0062] In a dry and clean reactor, add 50ml solvent 1,4-dioxane, then add 10mmol (II) compound, 15mmol elemental selenium, 15mmol N-bromosuccinimide and 25mmol pyridine successively, and the reaction system The reaction was stirred at 60°C for 12 hours under air atmosphere.
[0063] After the reaction was finished, the reaction system was cooled to room temperature, and then the solvent was removed by rotary evaporation with a rotary evaporator to remove the solvent from the mixture obtained after the reaction, and the residue was purified by 300-400 mesh silica gel column chromatography to obtain the target product. The yield was It is 84.7%, and the purity is 98.6% (HPLC).
[0064] NMR: 1 H NMR (CDCl 3 ,500MHz):δ7.48-7.51(m,4H),6.81(m,4H),3.81(s,6H);
[0065] 13 C NMR (CDCl 3 ,125MHz): δ160.1(2C), 135.4(4C), 122.0(4C), 114.7(2C), 55.3(2C).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com