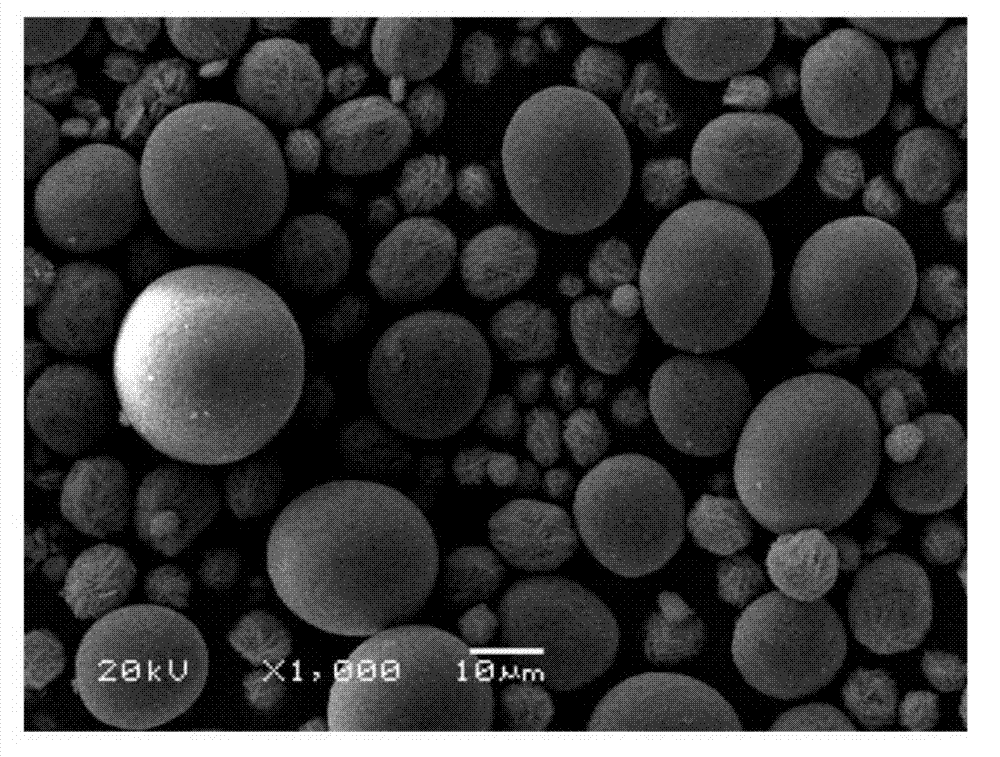
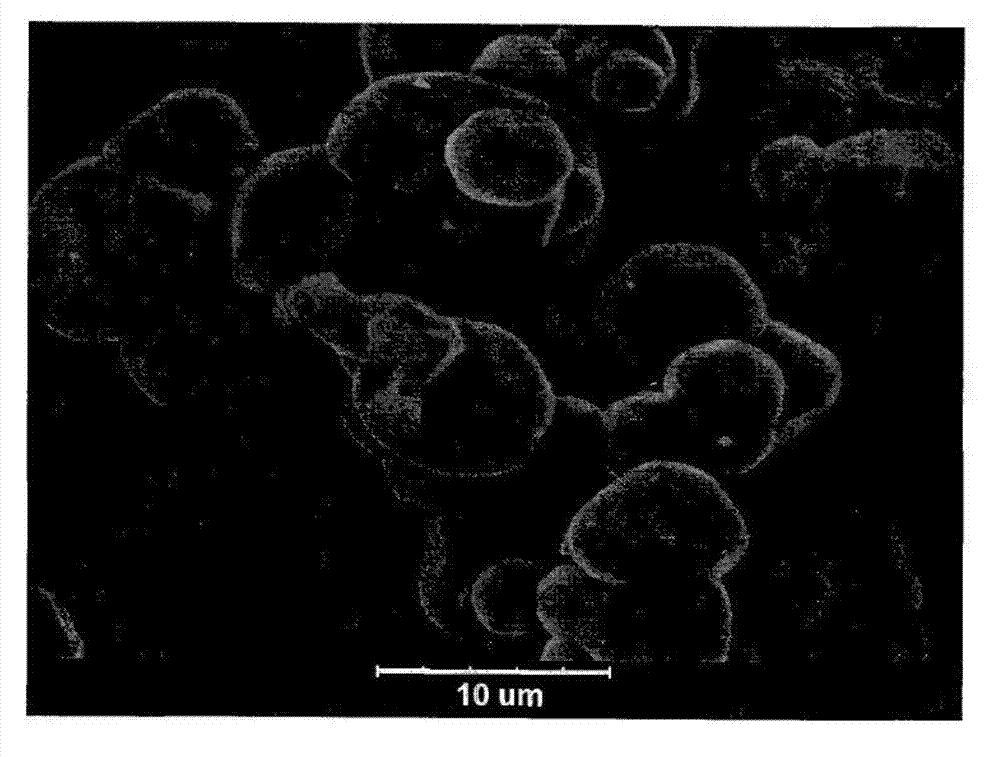
Preparation method of large-particle spherical cobaltosic oxide
A technology of tricobalt tetroxide and large particles, which is applied in the direction of cobalt oxide/cobalt hydroxide, etc., can solve the problems of insufficient sphericity, reaction, and low energy density of lithium-ion secondary batteries, so as to improve energy density, performance, and energy density high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Prepare 1.0mol / L cobalt sulfate (CoSO 4 ) aqueous solution;
[0028] (2) Prepare 4.0mol / L sodium hydroxide aqueous solution;
[0029] (3) Prepare a mixed solution of ethylenediamine and hydrazine hydrate with deionized water, and the mixed solution contains 0.3mol / L ethylenediamine and 0.05mol / L hydrazine hydrate;
[0030] (4) The above cobalt sulfate (CoSO 4 ) solution, sodium hydroxide solution and mixed solution were added to the reaction kettle at a flow rate of 250L / h, 100L / h and 50L / h respectively, the reaction temperature was controlled at 40°C, the reaction pH was 9, and the stirring speed was 700rpm / min, react for 12h to prepare the precursor;
[0031] (5) The above precursor was washed with deionized water, filtered, and then calcined at 800°C for 6 hours to obtain tricobalt tetroxide A.
Embodiment 2
[0033] (1) Prepare 1.0mol / L cobalt chloride (CoCl 2 ) aqueous solution;
[0034] (2) Prepare 5.0mol / L sodium hydroxide aqueous solution;
[0035] (3) Prepare a mixed solution of EDTA and sodium thiosulfate with deionized water, the mixed solution contains 0.5mol / L of EDTA and 0.1mol / L of sodium thiosulfate;
[0036] (4) The above cobalt chloride (CoCl 2 ) solution, potassium hydroxide solution and mixed solution were added to the reaction kettle at a flow rate of 150L / h, 150L / h and 40L / h respectively, the reaction temperature was controlled at 50°C, the reaction pH was 10, and the stirring speed was 900rpm / min, reacted for 13h to prepare the precursor;
[0037] (5) The above precursor was washed with deionized water, filtered, and then calcined at 800°C for 5 hours to obtain tricobalt tetroxide B.
Embodiment 3
[0039] (1) Prepare 2.0mol / L cobalt sulfate (CoSO 4 ) aqueous solution;
[0040] (2) Prepare 6.0mol / L potassium hydroxide aqueous solution;
[0041] (3) Prepare tartaric acid and hydrazine hydrate with deionized water to prepare a mixed solution containing 1.0 mol / L tartaric acid and 0.15 mol / L hydrazine hydrate;
[0042] (4) The above cobalt sulfate (CoSO 4 ) solution, potassium hydroxide solution and mixed solution were added to the reaction kettle at a flow rate of 100L / h, 120L / h and 30L / h respectively, the reaction temperature was controlled at 60°C, the reaction pH was 11, and the stirring speed was 1000rpm / min, react for 14h to prepare the precursor;
[0043](5) The above precursor was washed with deionized water, filtered, and then calcined at 700°C for 4 hours to obtain tricobalt tetroxide C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com