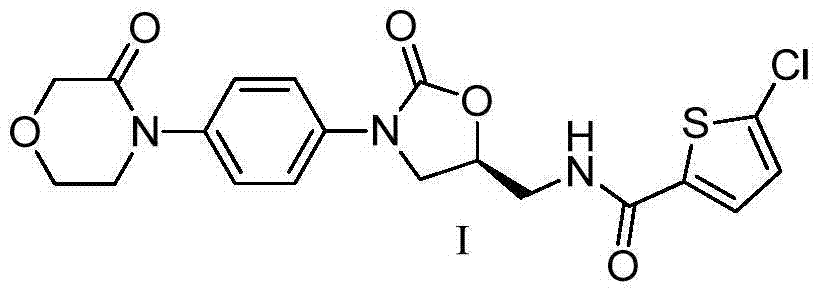
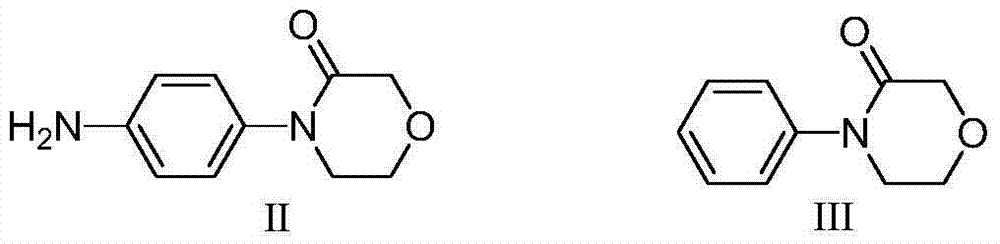
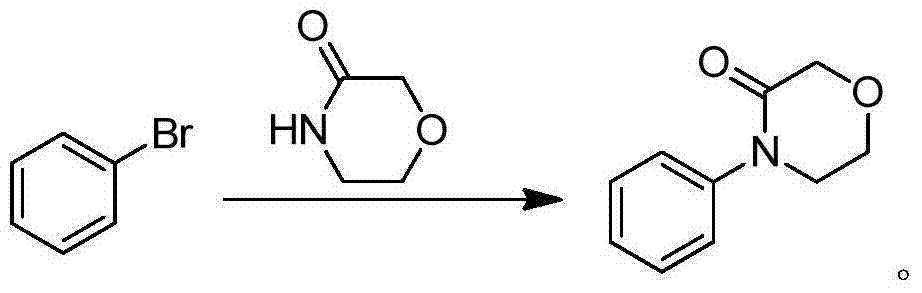
Preparation method of rivaroxaban intermediate
A technology for rivaroxaban and intermediates, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of difficult control of reaction conditions and cumbersome purification steps, and achieve the effects of low production cost, high product yield and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Suspend 24.0g (0.3mol) lithium tert-butoxide in 100mL dichloromethane, heat to reflux in a nitrogen atmosphere, add 18.6g (0.2mol) aniline in 100mL dichloromethane solution and 33.3g (0.2mol) 2-(2- Chloroethoxy)ethyl acetate solution in 100mL dichloromethane, after 1h dropwise addition, continue to reflux for 24h, add 100mL 5% dilute hydrochloric acid aqueous solution and stir for 10min, separate the water layer, and extract the water layer with 150mL dichloromethane. Combine the organic phases, wash with water (100mL×2), and anhydrous Na 2 SO 4 After drying, the solvent was removed under reduced pressure to obtain 32.1 g of white solid, yield 90.6%, m.p.113.5-114.5°C.
[0036] 1 H-NMR (CDCl 3 , 500MHz) δ: 3.77(2H,t,J=5.2Hz),4.04(2H,t,J=5.2Hz),4.35(1H,s),7.27(3H,m),7.42(2H,t,J =8.0Hz)
Embodiment 2
[0038] Suspend 3.4g (30.3mmol) of potassium tert-butoxide in 10mL of tetrahydrofuran, heat to reflux in a nitrogen atmosphere, add 1.9g (20.4mmol) of aniline in 10mL of tetrahydrofuran and 3.11g (0.02mol) of 2-(2-chloroethoxy ) 10mL tetrahydrofuran solution of methyl acetate, after 1h was added dropwise, the reflux reaction was continued for 24h. After the reaction was complete, the solvent was removed under reduced pressure. Add 10mL of 5% dilute sulfuric acid aqueous solution and stir for 10min, then extract with dichloromethane (15mL×3). The organic phases were combined, washed with water (10 mL×2), dried over anhydrous Na2SO4, and the solvent was removed under reduced pressure to obtain 3.0 g of white solid, yield 84.7%, m.p.113-114.5°C.
Embodiment 3
[0040] 2.9g (30.2mmol) sodium tert-butoxide was suspended in 10mL dichloromethane, heated to reflux in a nitrogen atmosphere, 1.9g (20.4mmol) aniline in 10mL dichloromethane solution and 3.40g (20.4mmol) 2-(2- Chloroethoxy)ethyl acetate solution in 10 mL dichloromethane, after 1 hour of dropwise addition, continue to reflux for 12 hours, add 10 mL of water and stir for 10 minutes, separate the water layer, and extract the water layer with 15 mL of dichloromethane. The organic phases were combined, dried over anhydrous Na2SO4, and dichloromethane was removed under reduced pressure to obtain 2.3 g of a white solid with a yield of 63.6%. m.p.113-114.5°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com