Immobilized nitrilase induced by self-aggregating short peptide and its preparation method and application
A technology of nitrilase and self-aggregation, applied in the fields of hydrolase, immobilized on/in organic carriers, chemical industry, etc., to achieve good substrate tolerance, save cumbersome operations, and facilitate long-term storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
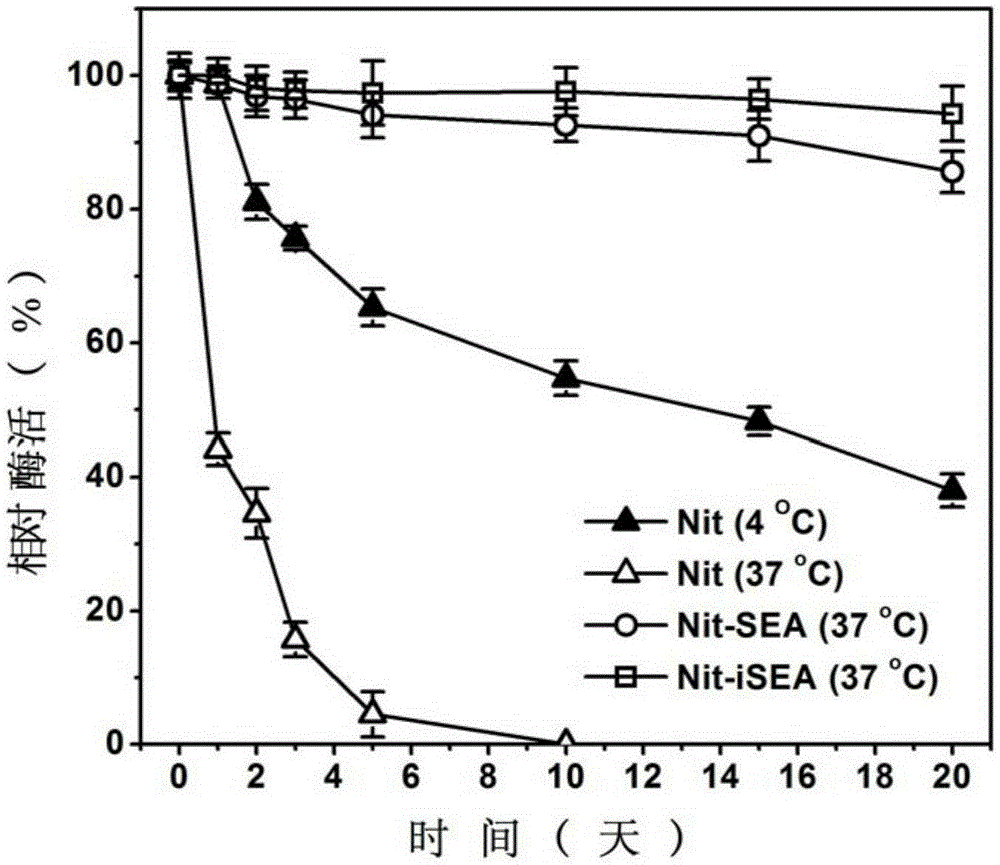
Examples
Embodiment 1
[0040] 1. Construction of expression vectors containing coding short peptides
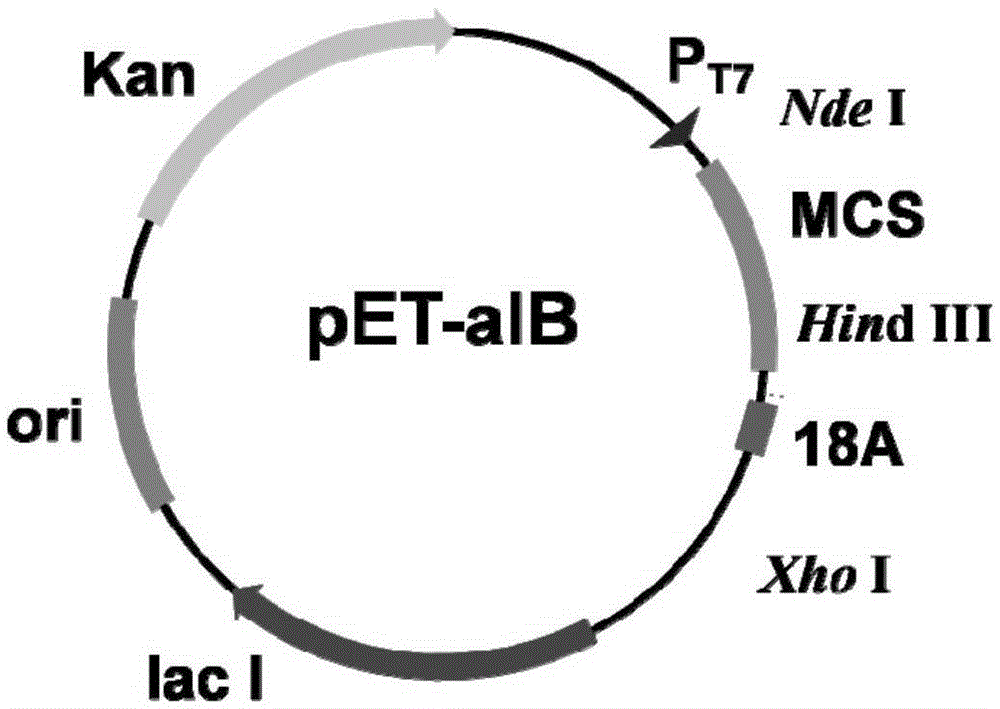
[0041] According to the amino acid sequence of PT-linker and 18A, the corresponding DNA sequence PT-linker+18A was synthesized in Gene Synthesis Company, and the HindIII restriction site was introduced upstream of the PT-linker+18A DNA sequence, and the XhoI enzyme site was introduced downstream. Plasmid pET-30a(+) was used as the basic plasmid, double-digested with HindIII and XhoI, connected into the corresponding PT-linker+18A sequence, transformed into E.coliDH5α competent cells, and passed colony PCR, enzyme digestion and sequencing Analyze and identify positive transformants to obtain plasmid pET-aIB, such as figure 1 shown.
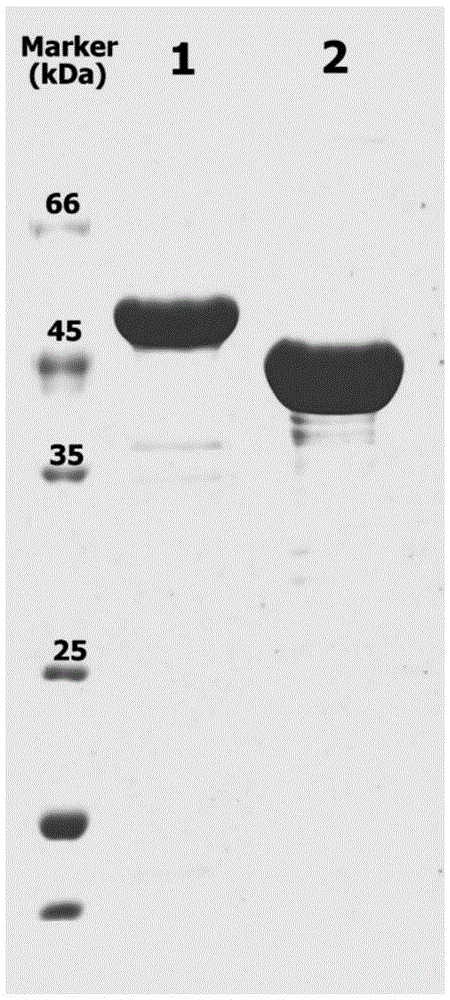
[0042] 2. Construction of engineering bacteria expressing nitrilase active aggregates in Escherichia coli cells
[0043] Using the genome of Alcaligenes faecalis JM3 as a template, under the action of primer 1 and primer 2, a 1 kb nitrilase gene fragment was amplified...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com