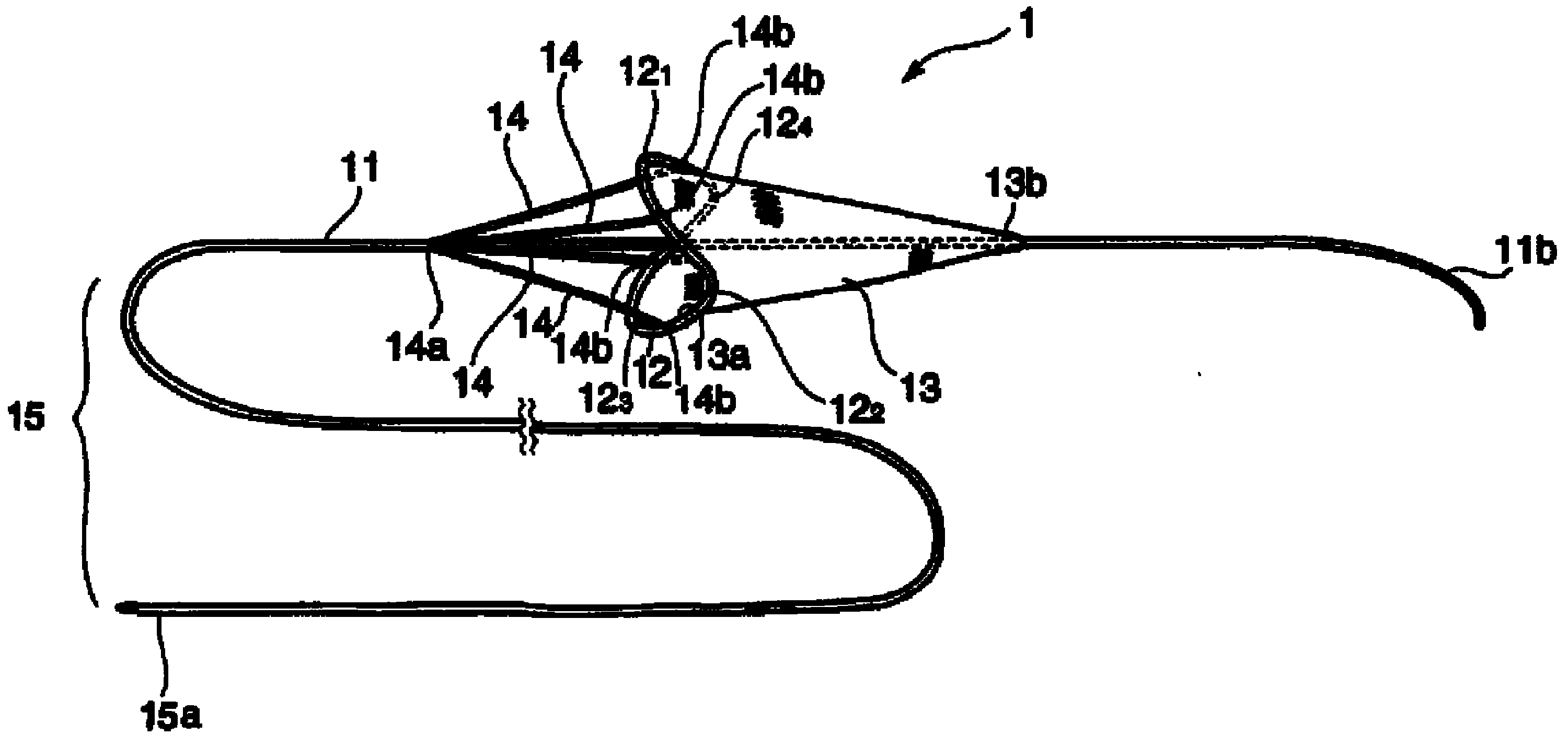
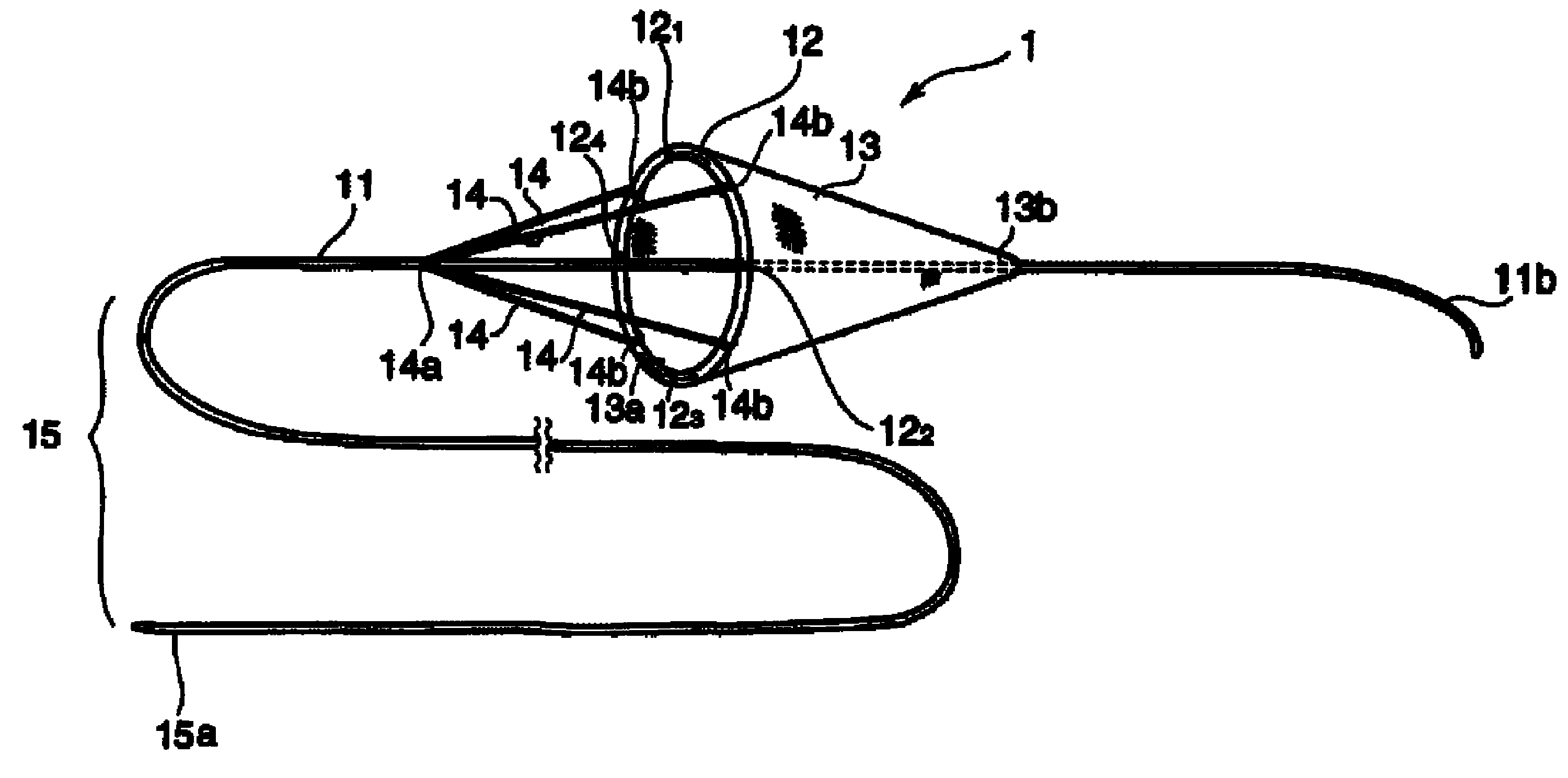
Instrument for capturing free thrombi
A device and thrombus technology, which is applied in the field of free thrombus capturing devices, can solve problems such as blood flow obstruction, and achieve the effect of reducing the burden and prolonging the usable time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] (Example 1: combination of amino-polyether modified silicone and argatroban)
[0078] Take 5 mmol of argatroban and add it to an eggplant-shaped flask, add 10 mL of dehydrated dimethylformamide (hereinafter referred to as "dehydrated DMF") to dissolve, and then ice-cool the eggplant-shaped flask, and simultaneously add 10 mL of 4N hydrochloric acid / 1, 4-Dioxane (Toyo Kasei Co., Ltd.), stirred for 1 hour. Next, the solvent was distilled off with a rotary evaporator, and then dried in a vacuum dryer overnight, and 25 mL of dehydrated DMF was added to the obtained dried product to prepare an argatroban hydrochloride / dehydrated DMF solution.
[0079] According to the portion shown in Table 1, take argatroban hydrochloride / dehydrated DMF solution and join in the two-necked flask, stir under ice-cold, add dicyclohexylcarbodiimide (hereinafter referred to as "DCC") / dehydrated DMF solution and 4-hydroxybenzotriazole (hereinafter referred to as "HOBt") / dehydrated DMF solution, ...
Embodiment 2~13
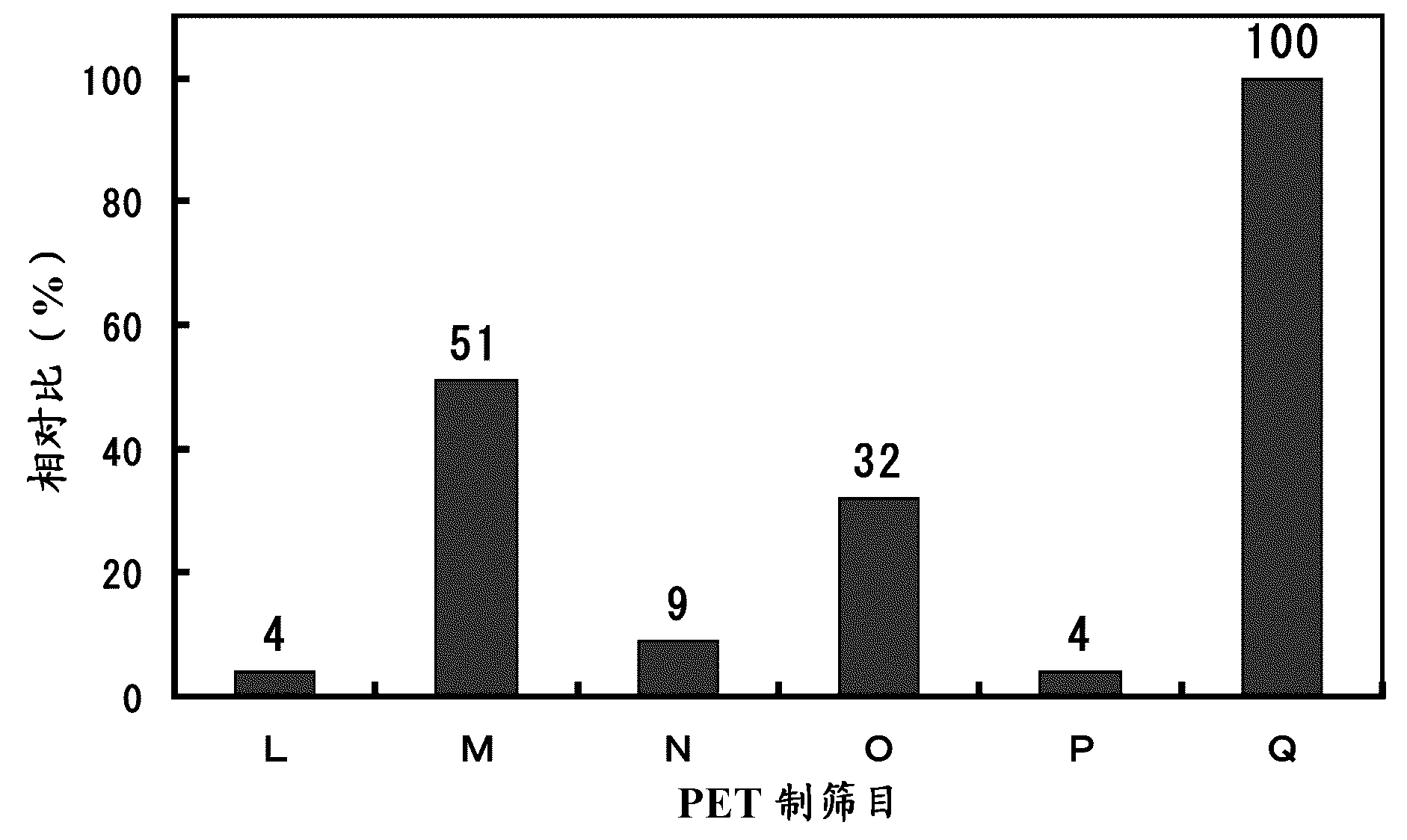
[0084] In addition to changing the molar ratio of DCC, HOBt and polyether-modified silicone (X-22-3939A) relative to argatroban hydrochloride and the volume ratio of dehydrated DMF and polyether-modified silicone, according to Example 1 The same method was used to obtain the compounds of Examples 2-13 respectively, and their antithrombin ability was determined. See Table 1 for the molar ratios of DCC, HOBt and polyether-modified silicone (X-22-3939A) relative to argatroban hydrochloride and the measurement results of the antithrombin abilities of the compounds of Examples 2-13.
[0085] [Table 1]
[0086]
[0087] It should be noted that for the polyether-modified silicone (X-22-3939A), the antithrombin ability was also measured, but the value was not different from the value of blank distilled water, and it can be confirmed that the polyether-modified silicone itself Does not have antithrombin ability.
[0088] (Measurement of the thrombin inhibition constant of embodime...
Embodiment 14
[0097] (Example 14: Combination of vinyl acetate-vinylpyrrolidone copolymer and argatroban)
[0098] Take 14.9g of tetrahydrofuran, 11.5g of vinyl acetate, 10.8g of N-vinylpyrrolidone, 0.028g of 2-aminoethanethiol and 0.016g of azobisisobutyronitrile into a screw bottle, seal it, and then irradiate with ultrasound for 10 minutes. Open the screw bottle temporarily, blow in argon gas for 10 minutes, seal it again, and then immerse the screw bottle in a hot water bath at 60°C for 1 hour while stirring, and then immerse it in a hot water bath at 70°C for 6 hours to make the acetic acid Copolymerization of vinyl esters and vinylpyrrolidone. 80 mL of methanol was added to the reaction liquid, the resulting solution was added to about 5 times the amount of diethyl ether, and the supernatant was removed. The cleaning operation of adding diethyl ether again and removing the supernatant was repeated three times, followed by drying under reduced pressure to obtain a vinyl acetate-vinylp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com