Preparation method of sodium nitroprusside injection
A technology of sodium nitroprusside injection and sodium nitroprusside, which is applied in the field of preparation of freeze-dried injections, can solve the problems of complex auxiliary agent process and increased risk of pollution, reduce the risk of impurity introduction, improve production efficiency, avoid The effect of reabsorbing moisture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
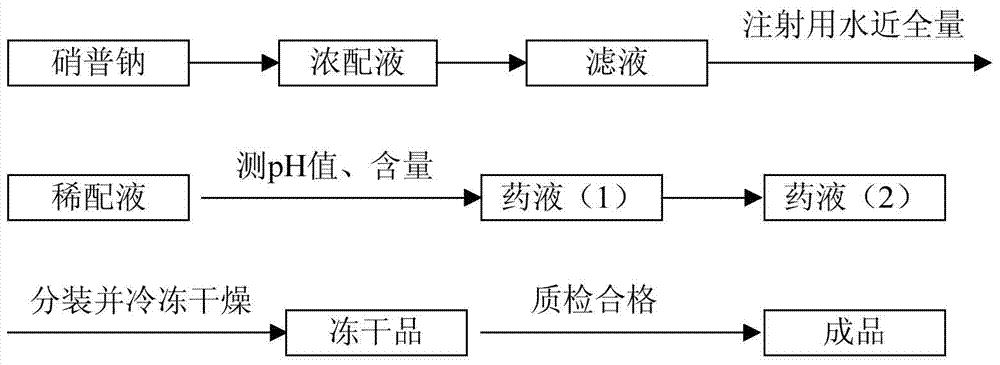
[0029] Process such as figure 1 . Aseptic control process measures include: materials entering the filling process are passed in after disinfection; sterile clean clothes are used after being sterilized by pulsating vacuum pure steam at 121°C for 30 minutes; moisture-resistant equipment for receiving and filling sterile liquid medicine All containers and utensils are sterilized by pulsating vacuum pure steam at 121°C for 30 minutes. Dry heat-resistant equipment and containers are all sterilized by dry heat at 220°C for 2 hours.
[0030] 1) Accurately weigh sodium nitroprusside according to the mass-volume ratio of adding 50g of sodium nitroprusside to 1000mL with water for injection, add water for injection to dissolve into a 20%-30% concentrated solution, add 0.1-0.2% activated carbon, and stir for 15 -25min, diatomaceous earth coarse filtration decarbonization;
[0031] 2) Add water for injection to 950-980mL, check the pH value, and make up the amount of water for injecti...
Embodiment 2
[0039] The aseptic control process is the same as in Example 1.
[0040] 1) Accurately weigh sodium nitroprusside according to the mass-volume ratio of adding 50g of sodium nitroprusside to 1000mL with water for injection, add water for injection to dissolve into a 30% concentrated solution, add 0.1wt% activated carbon, stir for 20min, diatomaceous earth Coarse filtration and decarbonization;
[0041] 2) Add water for injection to 980mL, check the pH value, and make up the amount of water for injection. Then pass through a 0.22μm microporous membrane filter. After fine filtration, samples were taken to detect content, pH, and bacterial endotoxin.
[0042] 3) The finely filtered solution is filled in sterile vials, half stoppered, placed in a freeze-drying device, and freeze-dried according to the freeze-drying curve:
[0043]Put the solution to be frozen after fine filtration in the freeze-drying equipment, the temperature of the equipment is reduced to -45 ° C, and then ke...
experiment example 1
[0057] Experimental Example 1 Routine physical and chemical performance testing
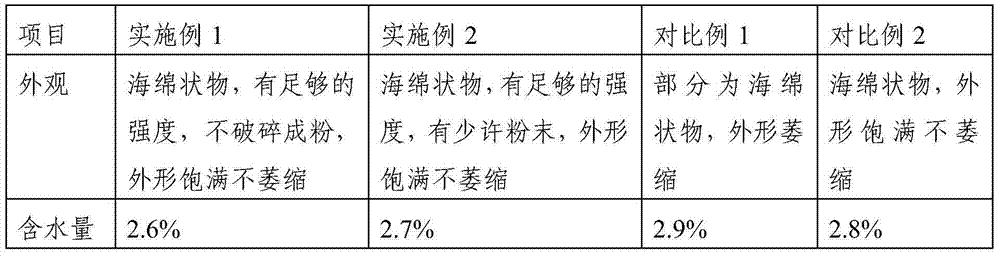
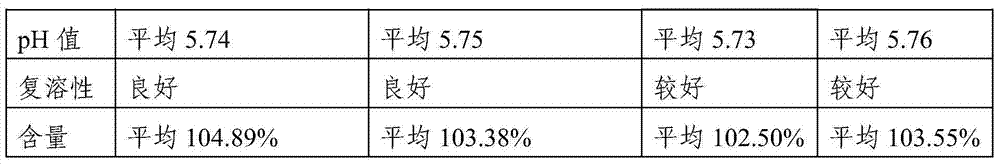
[0058] According to "Chinese Pharmacopoeia" 2000 edition two "sodium nitroprusside for injection" (P838), use ascorbic acid to react with sodium nitroferricyanide and sodium hydroxide to produce blue color, and inspect the product. Through the inspection to embodiment 1, embodiment 2, each four batches of samples of comparative example, the result is all in positive reaction. Conforms to the provisions of "Sodium Nitroprusside for Injection" in the 2000 edition of the Chinese Pharmacopoeia.
[0059] According to the provisions of "Sodium Nitroprusside for Injection" in Part Two of the "Chinese Pharmacopoeia" in 2000: take a sample, add water to make a solution containing 10 mg of Sodium Nitroprusside per 1 ml, and perform spectrophotometry (Appendix IV A in Part Two of the Chinese Pharmacopoeia in 2000) Measure, embodiment 1, embodiment 2, each four batches of samples of comparative example 1, 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com