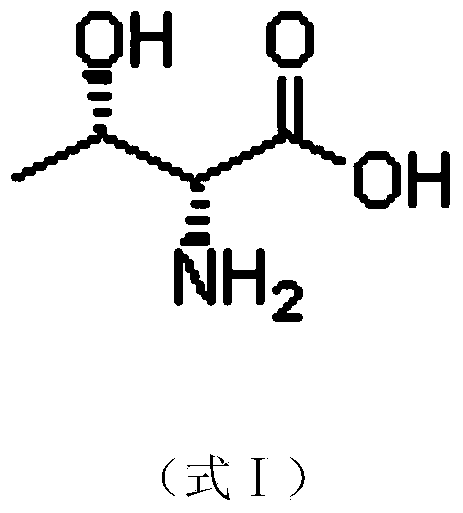
Preparation method of D-threonine
A technology of threonine and threonine copper, applied in the field of preparation of D-threonine, can solve the problems of low yield of DL-threonine, low total yield, high cost, etc., and achieve simple synthesis route, manual The effect of high sex content and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
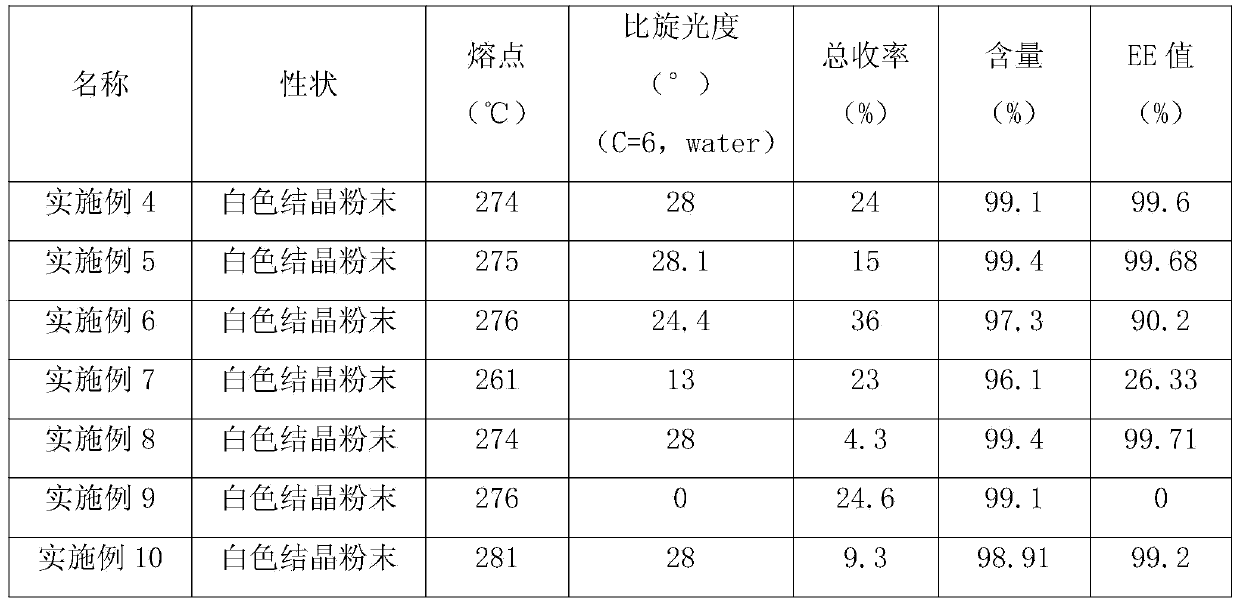
Examples
Embodiment 1
[0025] Embodiment 1: the preparation method of D-threonine, it comprises the following steps:
[0026] S1. Synthesis:
[0027] A. Dissolve glycine with a purity of 97% in 2mol / L sodium hydroxide solution, heat up to 65°C, react at a constant temperature for 6h, cool to room temperature, centrifuge, and dry to obtain copper glycine; wherein, glycine: hydroxide Sodium: the mass ratio of copper sulfate pentahydrate is 1:5:1.4;
[0028] B. Dissolve copper glycinate in water, add S-2-(diphenylhydroxymethyl)-tetrahydropyrrole, cool to 5°C, add acetaldehyde dropwise, react for 20 hours, D-threonine copper salt precipitates, separate solid Material, clean solid matter with 70% ethanol, remove lotion, get D-threonine copper salt; Wherein, copper glycinate: water: D-proline derivative: the mass ratio of acetaldehyde is 0.9:6:0.1: 1;
[0029] S2. Desalination: Dissolve D-threonine copper salt obtained in step S1 in 8% ammonia water, use hydrogen-type 732 cationic ammonia-type resin to...
Embodiment 2
[0031] Embodiment 2: the preparation method of D-threonine, it comprises the following steps:
[0032] S1. Synthesis:
[0033] A. Dissolve glycine with a purity of 98% in 2mol / L sodium hydroxide solution, heat up to 75°C, react at a constant temperature for 8h, cool to room temperature, centrifuge, and dry to obtain copper glycine; wherein, glycine: hydroxide Sodium: the mass ratio of copper sulfate pentahydrate is 1.05:6:1.6;
[0034] B. Dissolve copper glycinate in water, add N-triphenylmethyl-(L)-proline, cool to 10°C, add acetaldehyde dropwise, react for 28 hours, D-threonine copper salt precipitates, and separate solid matter , clean the solid matter with 80% ethanol, remove the lotion, and get D-threonine copper salt; wherein, copper glycinate: water: D-proline derivative: the mass ratio of acetaldehyde is 1:7:0.15:1.5 ;
[0035] S2. Desalination: Dissolve D-threonine copper salt obtained in step S1 in 12% ammonia water, use hydrogen-type 732 cationic ammonia-type res...
Embodiment 3
[0037] Embodiment 3: the preparation method of D-threonine, it comprises the following steps:
[0038] S1. Synthesis:
[0039] A. Dissolve glycine with a purity of 98.5% in 2mol / L sodium hydroxide solution, heat up to 70°C, react at a constant temperature for 7h, cool to room temperature, centrifuge, and dry to obtain copper glycine; wherein, glycine: hydroxide Sodium: the mass ratio of copper sulfate pentahydrate is 1.02:5.5:1.5;
[0040] B. Dissolve copper glycinate in water, add N-triphenylmethyl-(L)-proline, cool to 8°C, add acetaldehyde dropwise, react for 25 hours, D-threonine copper salt precipitates, and separates solid matter , clean solid matter with 76% ethanol, remove lotion, get D-threonine copper salt; Wherein, copper glycinate: water: D-proline derivative: the mass ratio of acetaldehyde is 0.95:6.8:0.12:1.3 ;
[0041] S2. Desalination: Dissolve D-threonine copper salt obtained in step S1 in 10% ammonia water, use hydrogen-type 732 cationic ammonia-type resin to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com