Substituted pyridine complex and preparation method thereof, and application of substituted pyridine complex as near ultraviolet photoluminescence material
A complex and pyridine technology, which is applied in the direction of luminescent materials, cadmium organic compounds, chemical instruments and methods, etc., can solve the problems of large damage and burn skin, etc., and achieve the effects of high yield, easy operation and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
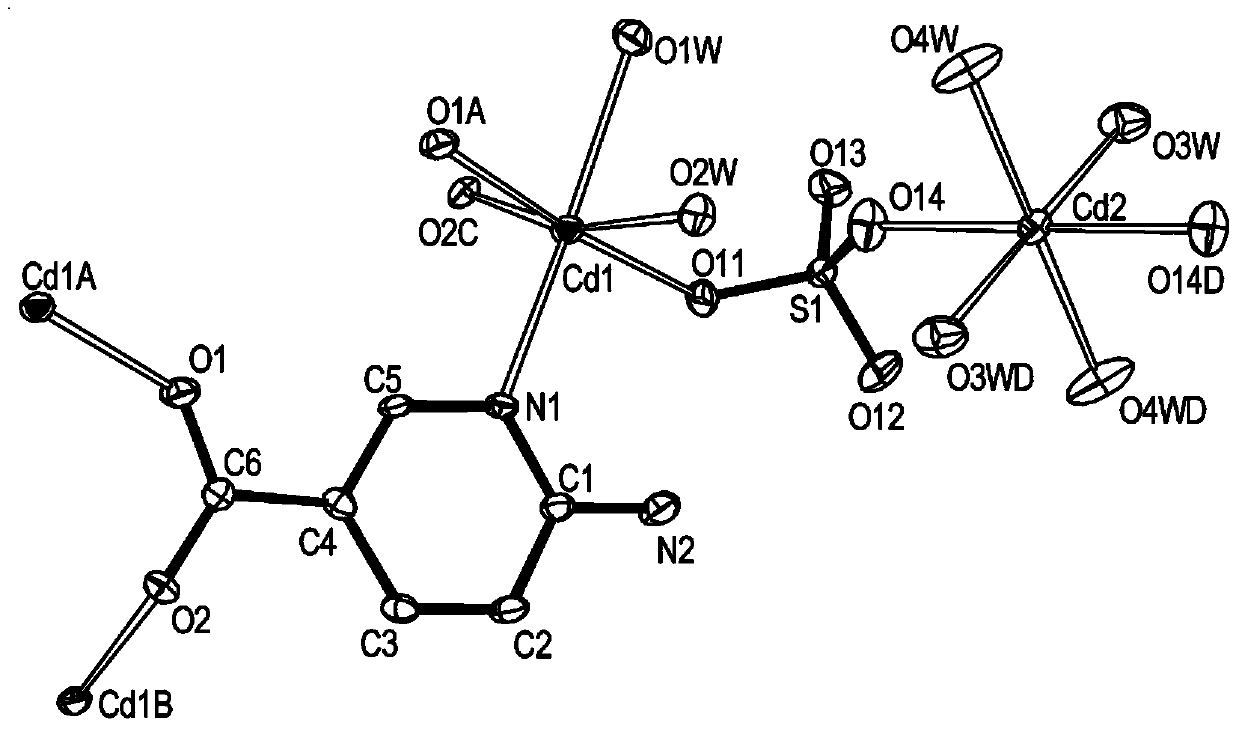
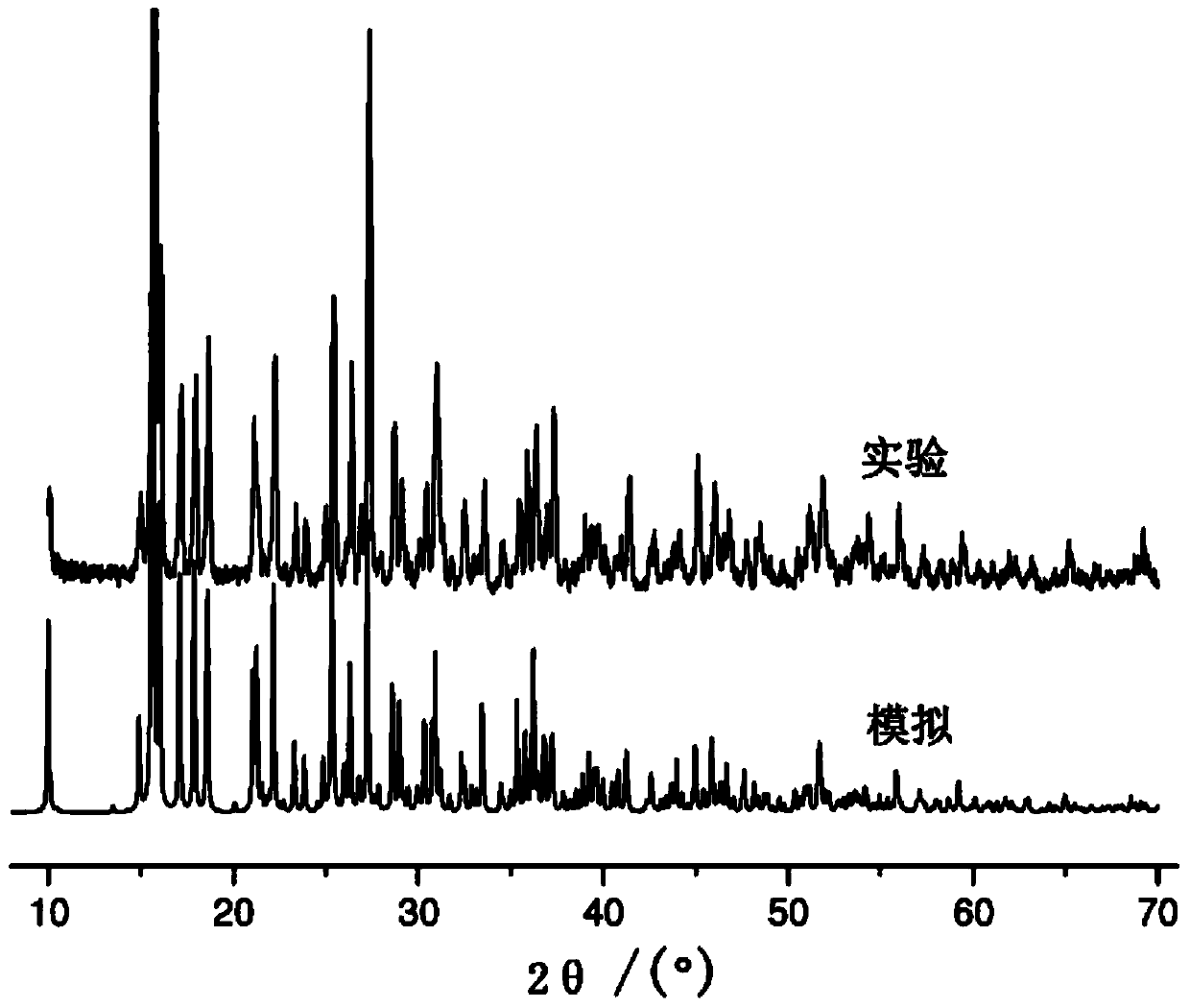
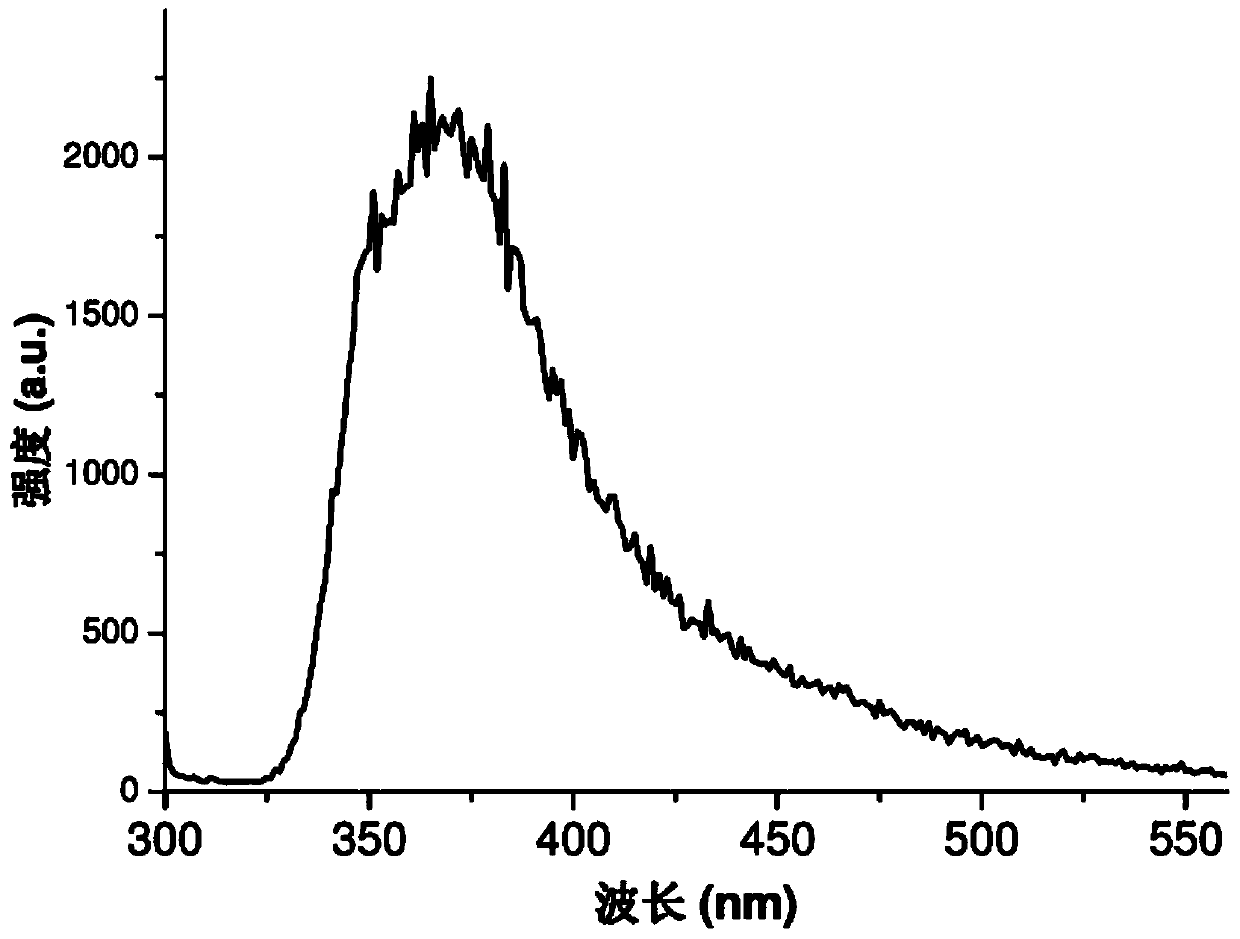
[0031] In conjunction with the accompanying drawings, a substituted pyridine complex of this embodiment, the chemical formula of the complex is Cd 3 (SO 4 ) 2 (C 6 h 5 N 2 o 2 ) 2 (H 2 O) 4 , the complex belongs to the monoclinic crystal system, the space group is P2(1) / c(No.14), and the unit cell parameters are a=16.934(7), b=10.365(4), c=7.251(3) α=γ=90°, β=92.875(9)°, Z=2. The schematic diagram of the molecular structure of the substituted pyridine complex is as follows: figure 1 Shown (the symmetric code indicates the symmetric relationship between the atoms). The substituted pyridine complex of this embodiment is to adopt 3CdSO 4 .8H 2 O, 6-aminopyridine-3-carboxylic acid (molecular formula is C 6 h 6 N 2 o 2 , after dehydrogenation is C 6 h 5 N 2 o 2 ) as a reactant, prepared by solvothermal method, 3CdSO 4 .8H 2 The molar ratio of O and 6-aminopyridine-3-carboxylic acid was 1:1. The maximum excitation wavelength of the substituted pyridine comple...
Embodiment 2
[0039] A substituted pyridine complex and its application as a near-ultraviolet photoluminescent material in this embodiment are basically the same as in Example 1, except that the preparation method of a substituted pyridine complex in this embodiment is as follows: 0.308g , 0.4mmol of 3CdSO 4 .8H 2 O, 0.110g, 0.8mmol of 6-aminopyridine-3-carboxylic acid, 4mL of water and 4mL of ethanol were successively loaded into a polytetrafluoroethylene reactor and heated. Under sealed conditions, the temperature was raised to 160°C at 12°C / h, and the temperature was constant. After 89 hours, the temperature was lowered to 30° C. at a rate of 4° C. / h to obtain the colorless flaky crystals, that is, the substituted pyridine complex.
Embodiment 3
[0041] A substituted pyridine complex and its application as a near-ultraviolet photoluminescent material in this embodiment are basically the same as in Example 1, except that the preparation method of a substituted pyridine complex in this embodiment is as follows: 0.308g , 0.4mmol of 3CdSO 4 .8H 2 O, 0.165g, 1.2mmol of 6-aminopyridine-3-carboxylic acid, 6mL of water and 3mL of ethanol were successively loaded into a polytetrafluoroethylene reactor and heated. Under sealed conditions, the temperature was raised to 180°C at 15°C / h, and the temperature was constant. After 96 hours, the temperature was lowered to 30° C. at a rate of 5° C. / h to obtain the colorless flaky crystals, that is, the substituted pyridine complex.
[0042] A substituted pyridine complex described in Examples 1 to 3, its preparation method and its application as a near-ultraviolet photoluminescence material, the fluorescence emitted by the substituted pyridine complex is near-ultraviolet light, which ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com