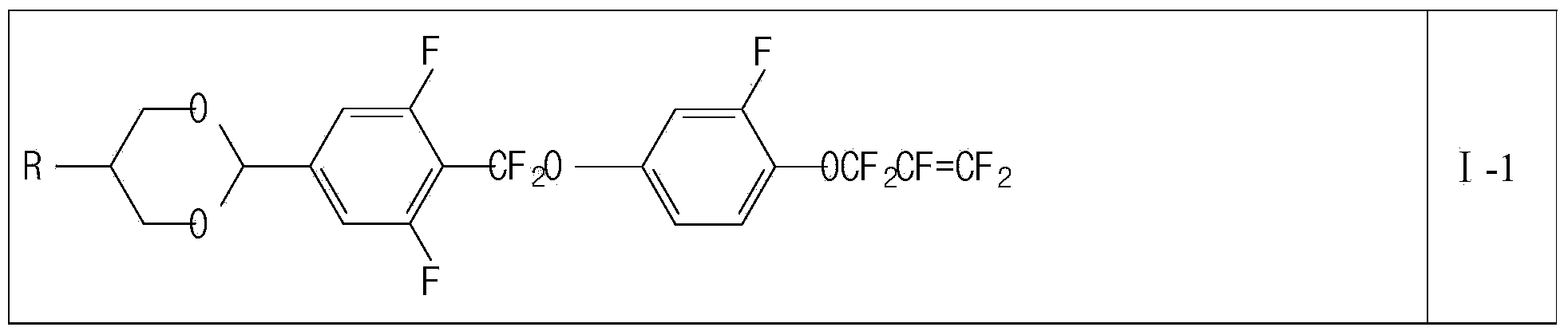
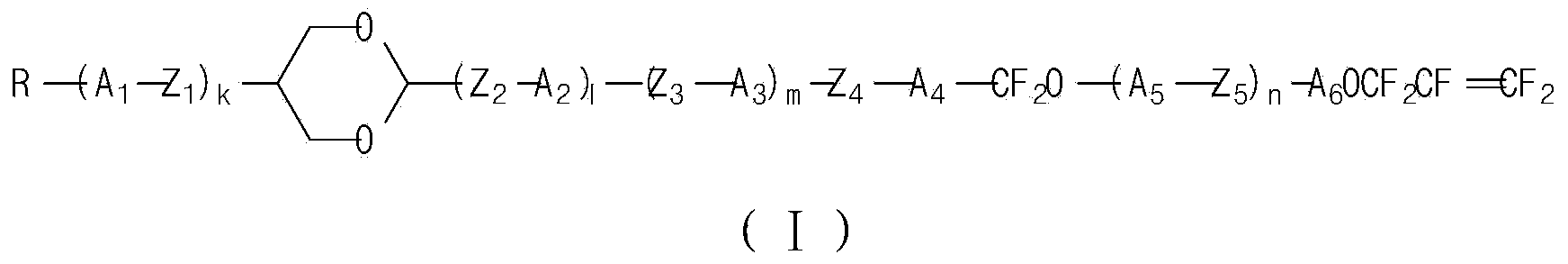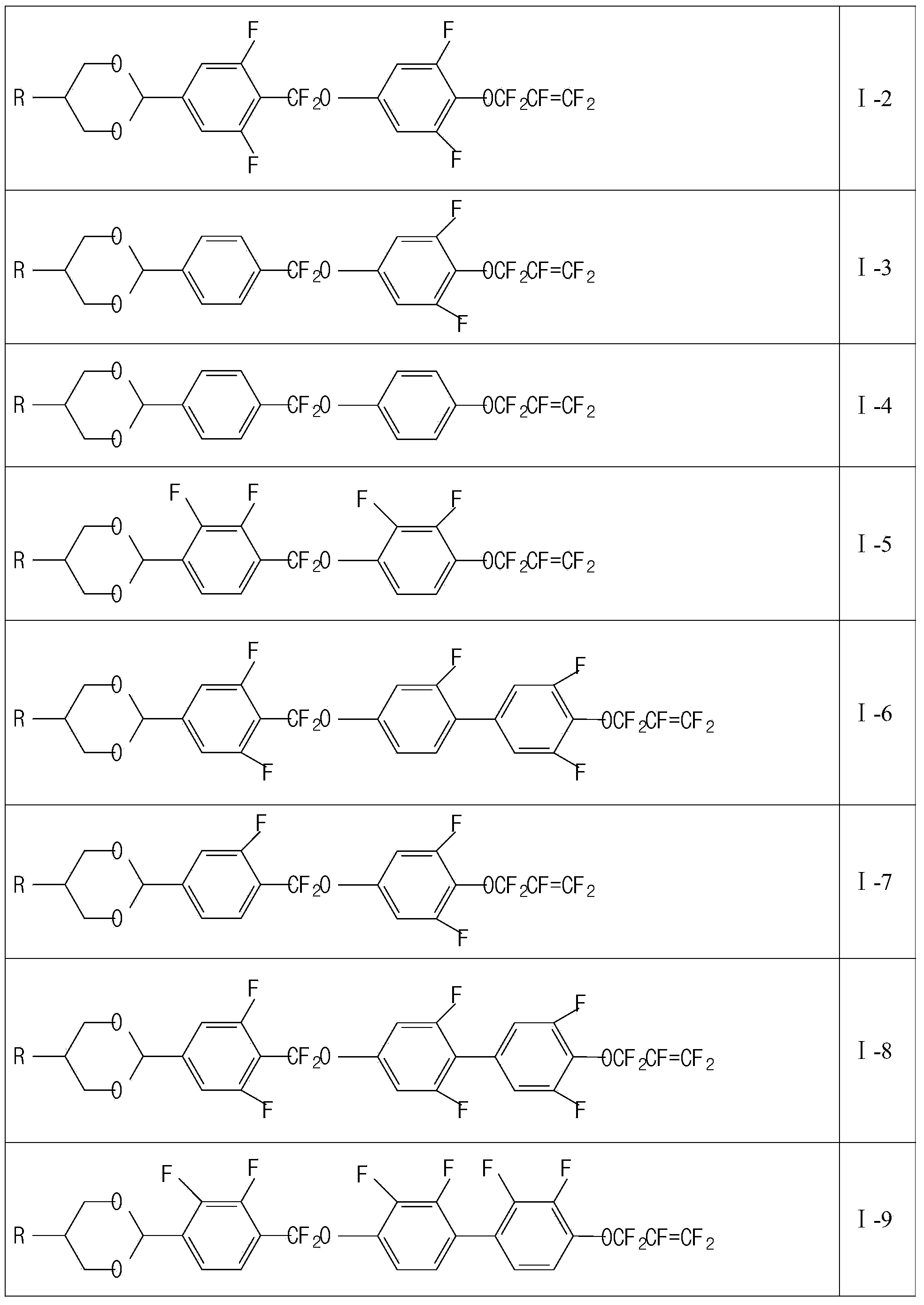Liquid crystal compound containing 1,4-dioxane and pentafluoro-allyloxy structure and liquid crystal composition thereof
A technology of pentafluoroallyloxy and liquid crystal compounds, applied in liquid crystal materials, organic chemistry, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] BYLC-01
[0066] The synthetic route for preparing compound BYLC-01 is as follows:
[0067]
[0068] Synthesis of Step 1-1 Compound 3
[0069] Add 40g (0.338mol) of compound 1, 50g (0.354mol) of compound 2, 2.8g of p-toluenesulfonic acid, 1.0g of 2,6-di-tert-butyl-p-cresol, 0.2L of toluene into a 500ml three-necked flask with a water separator , start stirring, heat up to 125°C to reflux and divide the water, after timing the reaction for 8 hours, pour the reaction solution into 500ml of water, stir, separate the liquids, extract the water phase with toluene, wash the organic phase three times with water, separate the liquids, and combine all the organic phases Dry with anhydrous sodium sulfate for 0.5h, filter with suction, combine the filtrates to concentrate and dry the solvent, add 4 times of petroleum ether to dissolve through a silica gel column, concentrate and dry, add 2 times of ethanol to dissolve and recrystallize, and filter with suction to obtain 61.5...
Embodiment 2
[0081] BYLC-02
[0082] The synthetic route for preparing compound BYLC-02 is as follows:
[0083]
[0084] Synthesis of Step 1-1 Compound 3
[0085] Add 80g (0.4mol) of compound 1, 85g (0.598mol) of compound 2, 4.5g of p-toluenesulfonic acid, 2.0g of 2,6-di-tert-butyl-p-cresol, 0.2L of toluene into a 500ml three-necked flask with a water separation device , start stirring, heat up to 125°C to reflux and divide the water, after timing the reaction for 10 hours, pour the reaction solution into water, stir, separate the liquids, extract the water phase with toluene, combine the organic phases and wash them three times, and dry the organic phases with anhydrous sodium sulfate 0.5h, suction filtration, combined filtrate concentrated dry solvent, added 4 times petroleum ether to dissolve through silica gel column, concentrated dried, added 2 times ethanol for recrystallization, obtained 93g light yellow solid compound 3, GC: 99.43%, yield: 71.7% .
[0086] Synthesis of step...
Embodiment 3
[0097] BYLC-03
[0098] The synthetic route for preparing compound BYLC-03 is as follows:
[0099]
[0100] Synthesis of Step 1-1 Compound 3
[0101] Add 50g (0.423mol) of compound 1, 92.3g (0.423mol) of compound 2, 4.2g, p-toluenesulfonic acid, 1.8g of 2,6-di-tert-butyl-p-cresol, 0.2L into a 500ml three-necked flask with a water separation device Toluene, start stirring, heat up to 125°C to reflux and divide water, time the reaction for 5 hours, pour the reaction solution into water, stir, separate the liquid, extract the water phase with toluene, combine the organic phase and wash three times with water, and use anhydrous sodium sulfate for the organic phase Dry for 0.5h, filter with suction, combine the filtrates to concentrate and dry the solvent, add 4 times of petroleum ether to dissolve through a silica gel column, concentrate and dry, add 2 times of ethanol for recrystallization, and obtain 101.2g of light yellow solid compound 3, GC: 99.43%, yield: 75.3%.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com