Method for loading nanocrystalline metal oxide or nanocrystalline metal materials by porous carbon
A technology of nano metal and metal material, applied in the field of porous carbon supported nano metal oxide or nano metal material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Porous carbon loaded nano-Fe
[0025] Synthetic raw materials: glucose, urea, Fe(NO 3 ) 3? 9H 2 O (iron nitrate)
[0026] (1) Weigh 10 g glucose, 1 g urea and 0.1 g Fe(NO 3 ) 3? 9H 2 O in a 100 mL beaker, then place the beaker in a heatable magnetic stirrer. The temperature of the magnetic stirrer was raised to 100° C., and the stirring was continued for 60 min until the medicine in the beaker was in a molten state.
[0027] (2) From the molten liquid mentioned in (1), take out a part of the solution and put it in a 120°C oven as sample A, and put the other part of the solution into a high-temperature reaction kettle and put it in a 120°C oven as sample B, and react for 48 hours. A dark brown bulky solid was obtained, and sample B was dark brown dense solid.
[0028] (3) Grind the sample A and sample B obtained in (2) with a mortar, and divide them into two crucibles, and then put them under N 2 Heat treatment at 550°C for 7 hours under protection to...
experiment example 2
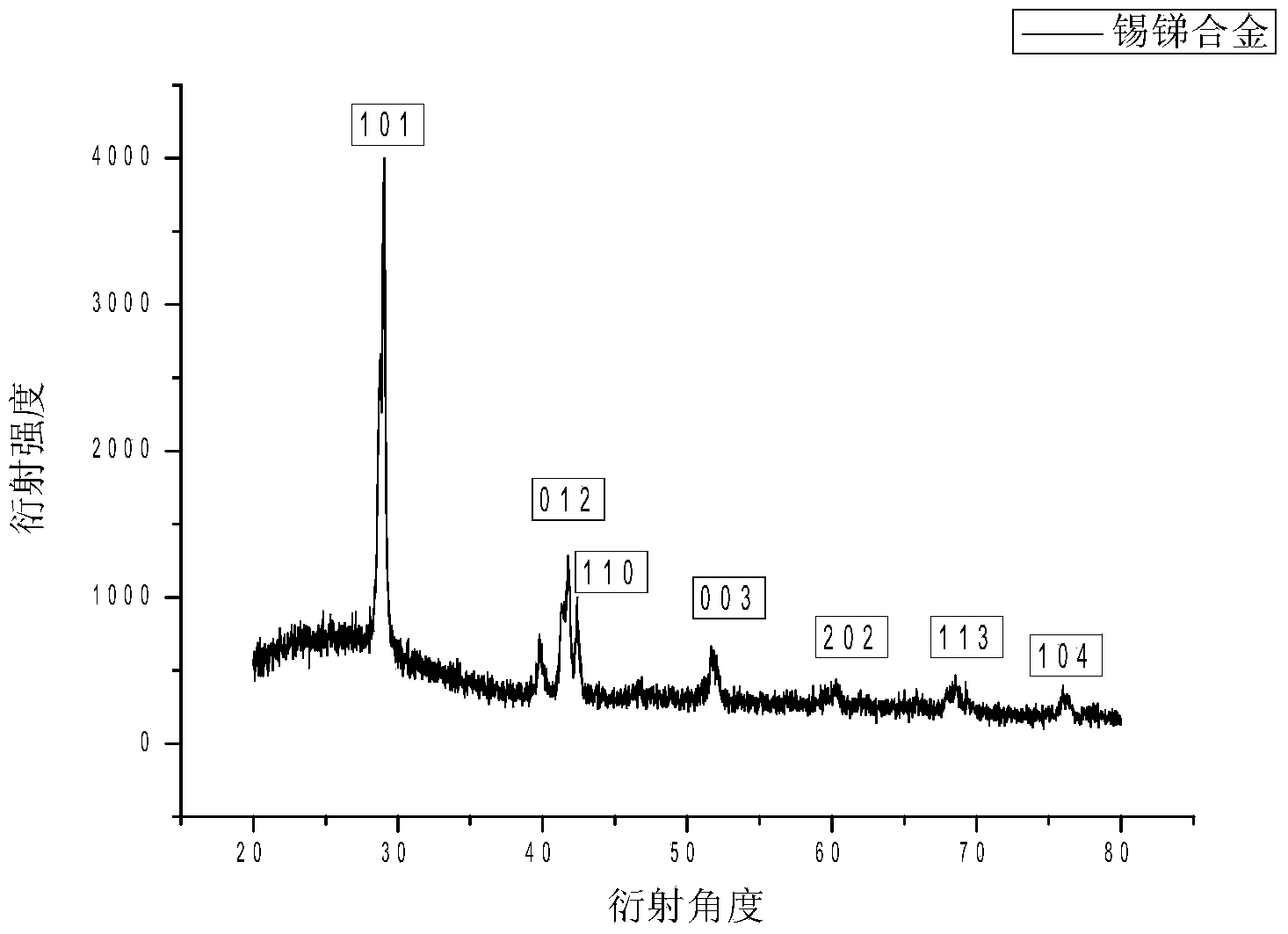
[0031] Experimental example 2: Porous carbon supported nano-SnSb alloy
[0032] Synthetic raw materials: glucose, urea, SnCl 2? 2H 2 O (Stannous Chloride), SbCl 3 (antimony chloride)
[0033] (1) Weigh 1 g glucose, 10 g urea, 0.3 g SnCl 2? 2H 2 O and 0.3 g SbCl 3 In a 100 mL beaker, then place the beaker in a heatable magnetic stirrer. The temperature of the magnetic stirrer was raised to 220°C, and the stirring was continued for 60 min until the drug in the beaker formed a molten state.
[0034] (2) Afterwards, put the beaker into an oven at 250°C and react for 1 hour to obtain a dark brown puffy solid.
[0035] (3) Grind the product obtained in (2) with a mortar and put it in a crucible. The product obtained by the reaction was heated at 250°C in 5% H 2 / N 2 Under the condition of heat treatment for 24 hours, the porous carbon-loaded nano-SnSb alloy is obtained.
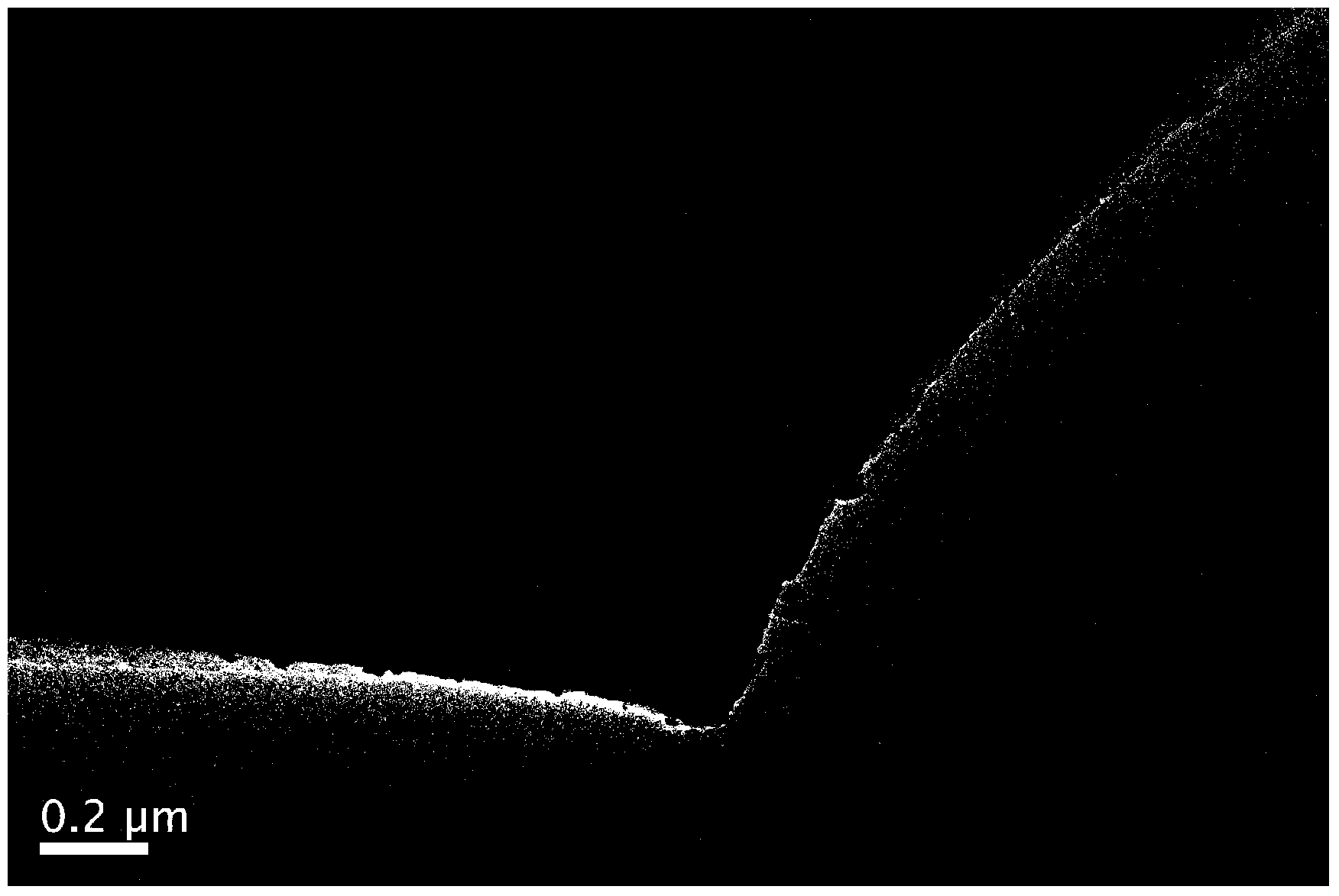
[0036] figure 1 It is a TEM image of nano-SnSb alloy supported on porous carbon. In the figure, it ...
experiment example 3
[0038] Experimental example 3: Porous carbon supports nano-Pd
[0039] Synthetic raw materials: fructose, urea, Pd(NO 3 ) 2? 2H 2 O (palladium nitrate)
[0040] (1) Weigh 100 g fructose and 1 g urea into a 100 mL beaker, then place the beaker in a heatable magnetic stirrer. The temperature of the magnetic stirrer was raised to 180°C, and the stirring was continued for 10 min until the drug in the beaker formed a molten state.
[0041] (2) Weigh 0.1 g Pd(NO 3 ) 2? 2H 2 Add O into the molten liquid described in (1), and keep stirring for 8 min until the solution is clear. Afterwards, the molten liquid was added into a hydrothermal reaction kettle and placed in an oven at 180° C., and reacted for 24 hours to obtain a dark brown solid.
[0042] (3) Grind the product obtained in (2) with a mortar and put it in a crucible. The product obtained by the reaction was heated at 1100°C in 5%H 2 / N 2 After heat treatment for 3 hours under the same conditions, porous carbon-suppo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com