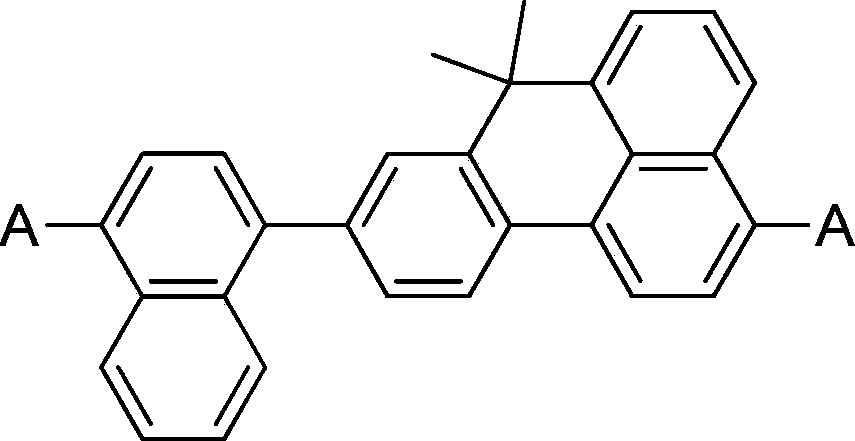
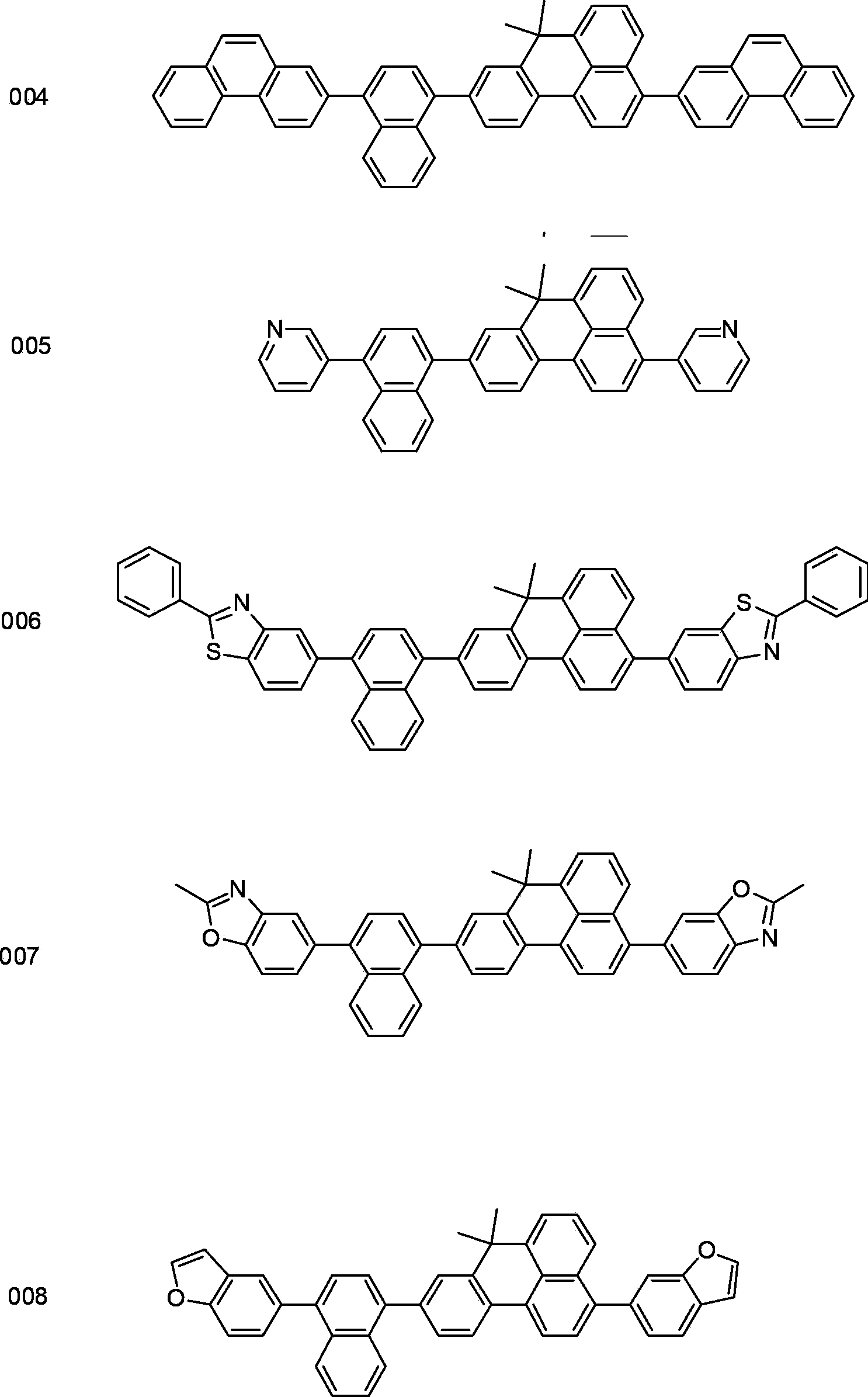
Organic blue luminescent material, and preparation method and application thereof
A blue light-emitting material and a technology for light-emitting materials, which are applied in the field of organic blue light-emitting materials and their preparation, can solve the problem that blue light materials cannot meet industrial production, and achieve the effects of improving light-emitting efficiency, low preparation cost, and good film-forming performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
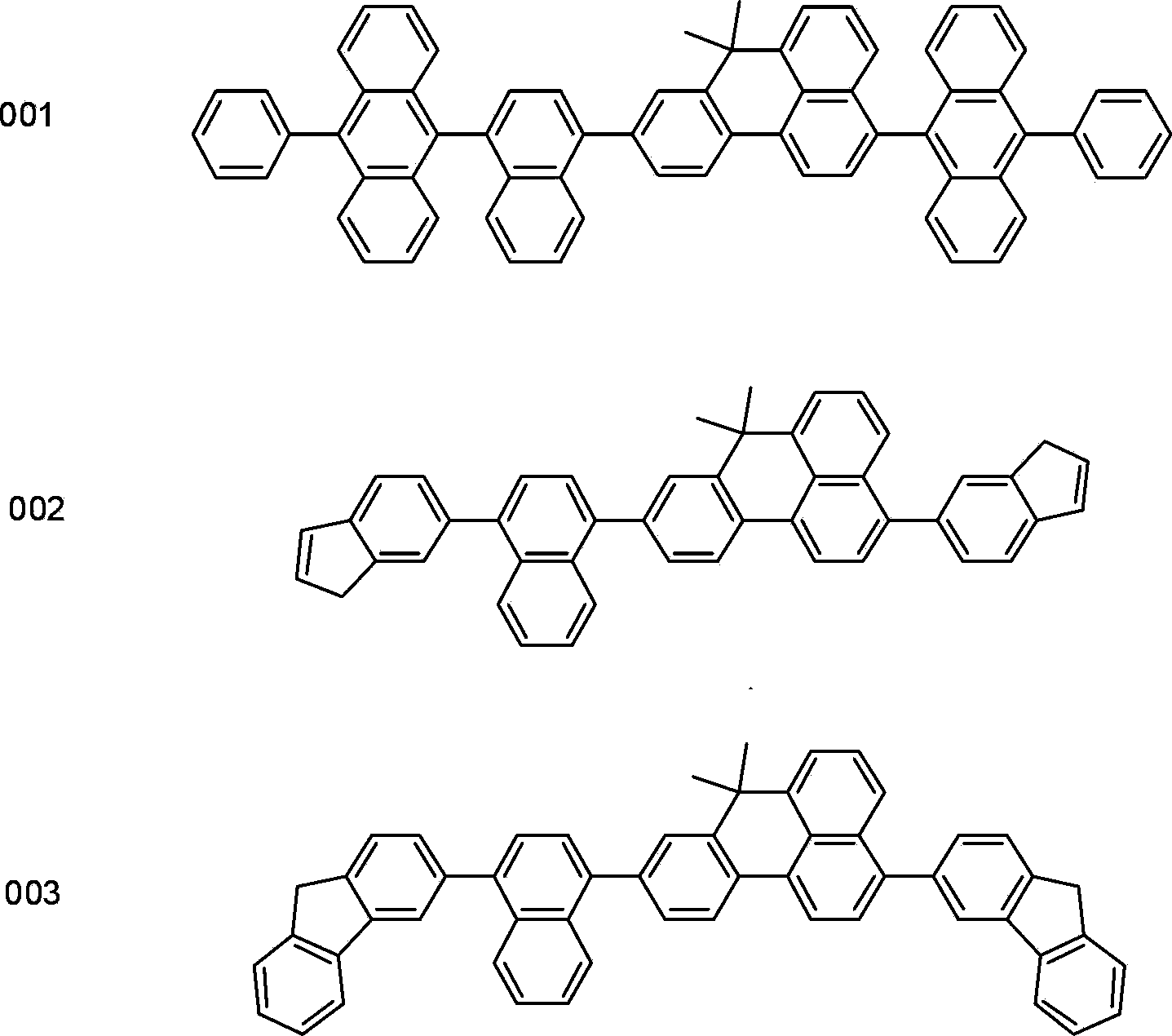
[0031] Embodiment 1: the synthesis of compound 001
[0032] The specific synthetic route is shown in the following formula:
[0033]
[0034] 26.41g of 3-bromo-9-(4-bromonaphthyl)-7,7-dimethyl-7H-benzanthracene, 44.72g of (10-phenyl-9-anthracenyl)boronic acid, 20g of sodium carbonate, Put 250ml of tetrahydrofuran and 125ml of water into a three-necked flask, degas, add 0.9g of tetrakis(triphenylphosphine)palladium, heat up to 70°C, reflux for 30 hours, cool to room temperature, after the solid precipitates, suction filter, filter cake through water, After washing with ethanol and ether, drying to obtain 56.17 g of asymmetric benzanthracene derivatives with the chemical structure formula 001, the yield is over 92%, and the HPLC purity is over 98%. Mass Spectrum: Calculated 875.10; Found 875.05. Elemental analysis: calculated value C: 94.70%; H: 5.30%; test value C: 94.73%; H: 5.27%.
Embodiment 2
[0035] Embodiment 2: the synthesis of compound 002
[0036] The specific synthetic route is shown in the following formula:
[0037]
[0038] Add 26.41g of 3-bromo-9-(4-bromonaphthyl)-7,7-dimethyl-7H-benzanthracene, 22.32g of 6-indenylboronic acid, 20g of sodium carbonate, 250ml of tetrahydrofuran and 125ml of water into three ports bottle, degassed, added 0.9 g of tetrakis(triphenylphosphine)palladium, raised the temperature to 100°C, refluxed for 5 hours, cooled to room temperature, and after the solid precipitated, suction filtered, the filter cake was washed with water, ethanol and ether, and dried After drying, 25.18 g of asymmetric benzanthracene derivatives of the chemical structural formula 002 were obtained, with a yield of more than 93% and an HPLC purity of more than 98%. Mass spectrum: calculated value 598.77; found value 598.75. Elemental analysis: calculated value C: 94.28%; H: 5.72%; test value C: 94.26%; H: 5.74%.
Embodiment 3
[0039] Embodiment 3: the synthesis of compound 003
[0040] The specific synthetic route is shown in the following formula:
[0041]
[0042] Add 26.41g of 3-bromo-9-(4-bromonaphthyl)-7,7-dimethyl-7H-benzoanthracene, 29.10g of 3-fluorenylboronic acid, 20g of sodium carbonate, 250ml of tetrahydrofuran and 125ml of water into three ports bottle, degassed, added tetrakis(triphenylphosphine)palladium 0.9g, raised the temperature to 75°C, refluxed for 24 hours, cooled to room temperature, after the solid precipitated, suction filtered, the filter cake was washed with water, ethanol and ether, and dried Dry to obtain 28.44 g of asymmetric benzanthracene derivatives with chemical structural formula 003, the yield is over 90%, and the HPLC purity is greater than 98%. Mass Spectrum: Calculated 698.89; Asserted 698.87. Elemental analysis: calculated value C: 94.52%; H: 5.48%; test value C: 94.50%; H: 5.50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com