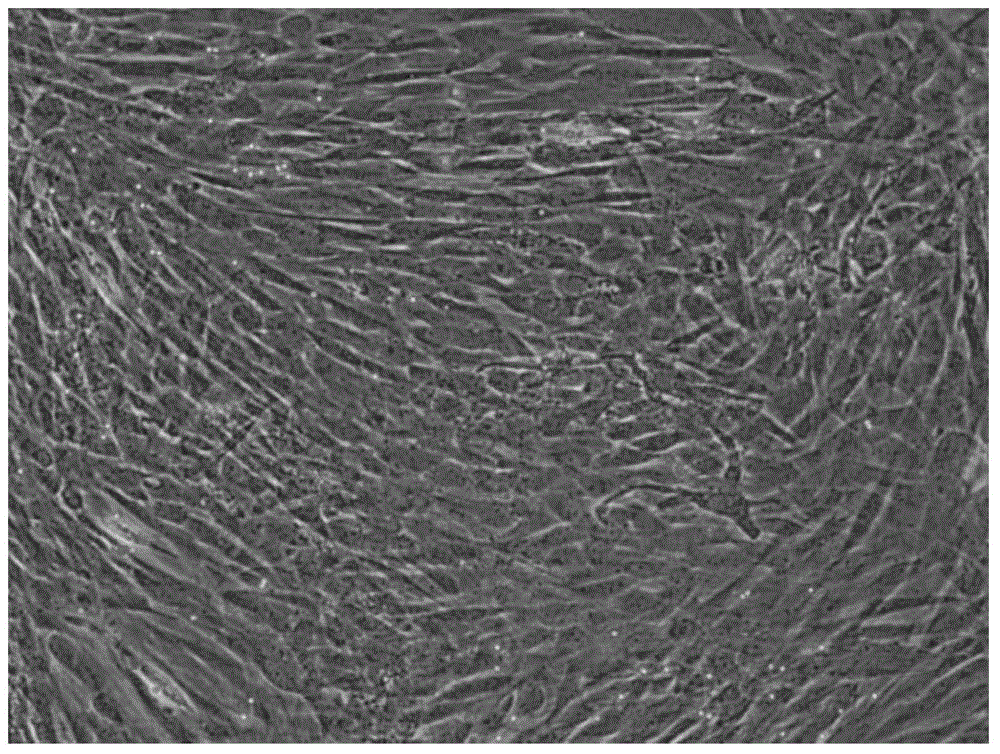
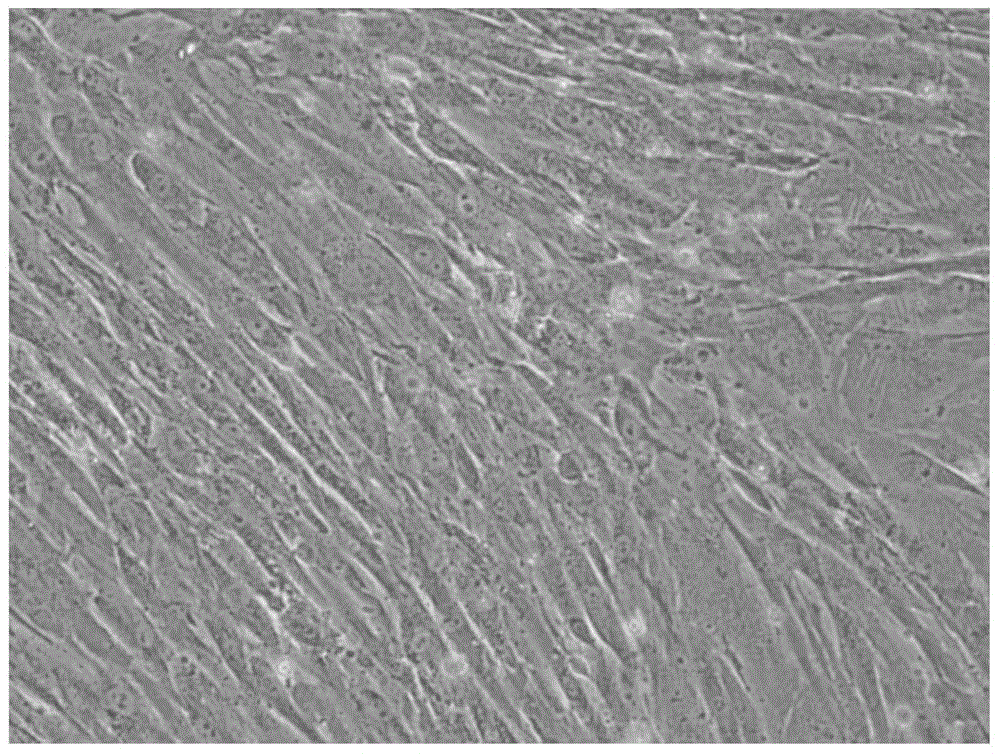
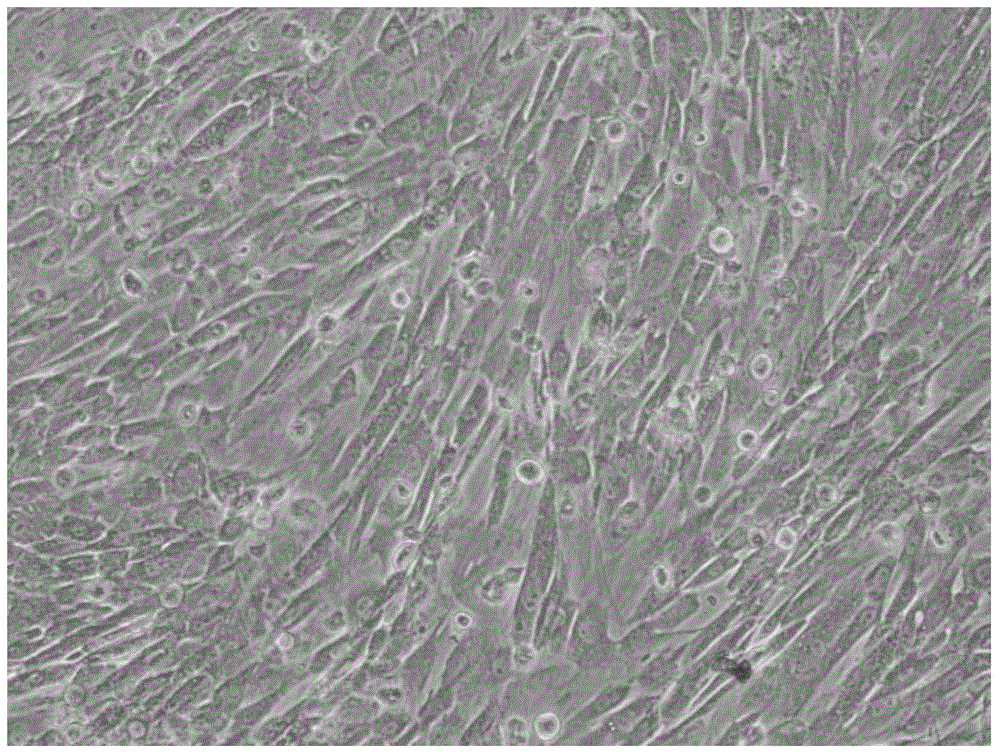
Serum-free medium for culturing placental mesenchymal stem cells
A serum-free culture medium and stem cell technology, applied in the field of cell culture compositions, can solve the problems of increased cost and risk, lack of culture medium, etc., and achieves improved in vitro separation and preparation efficiency, high growth and proliferation rate, and increased number of Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Combination experiment of cytokines in serum-free medium
[0031] Selected growth factors: Recombinant human fibroblast growth factor 2 (hFGF), human recombinant insulin (I), human recombinant growth hormone (hGH), human recombinant transferrin (TF) and BMP-4 concentrated solution The liquid is sterilized by filtration through a sterile 0.22μm water phase membrane or organic phase membrane (this step does not need to be carried out if a sterile concentrate is purchased), and refrigerated or frozen for later use.
[0032] Design different concentrations for different growth factors (see Table 1) and add them to the culture medium and find the optimal effect concentration of the growth factor according to the growth curve. After concentration screening experiments, different growth factors with optimal concentrations were combined (see Table 2), and their effects on the growth of stem cells (see Table 3) were investigated to obtain the optimal growth factor combinati...
Embodiment 2
[0042] Take the chorionic membrane on the fetal side of the placenta, cut it into small pieces, collect the cells after digestion with 0.13v / v% collagenase, and adhere to the cell culture medium (containing DMEM, 10v / v% fetal bovine serum, 2mML-glutamine) to cultivate.
[0043] 1. Cells are cultured adherently in a petri dish, and the culture medium and suspended cells are discarded after 48 hours. Change the medium every 2-3 days. When the cell fusion rate reaches 90%, tryspin-EDTA digests and harvests the cells. After the harvested cells are washed once with PBS, they are re-inoculated and added with fresh medium for subculture.
[0044] 2. Subculture the harvested cells. The medium used is DMEM as the basic solution, and also contains 2mML-glutamine, 55uMb-mercaptoethanol, 1mM sodium pyruvate, 1v / v% non-essential amino acids (Gibco), 10μg / ml insulin, 0.1μg / ml transferrin, 200μg / ml glutathione, 0.1ng / mlBMP-4, 10ng / mlFGF-2 and 3ng / ml hGH) serum-free medium.
Embodiment 3
[0046] For the determination of the surface markers of cells cultured in serum and serum-free media, flow cytometry was used to determine the ratio of different labeled cell surface antigens in mesenchymal stem cells cultured and harvested in different culture systems (see Table 3).
[0047] table 3
[0048]
[0049] CD90 in primary placental fetal chorionic cells + , CD73 + And CD105 + The average cell numbers were 99.06%, 99.98%, and 99.10%, respectively, indicating that the primary adherent spindle cells have significant mesenchymal cell characteristics. After 2 passages, adherent culture, in a serum medium, CD90 + And CD105 + The cells are significantly reduced, and CD90 in serum-free medium + And CD105 + Cells can still exist in a relatively high ratio, maintaining the characteristics of mesenchymal stem cells. According to the above comparison, it can be concluded that the cells isolated from the chorion on the fetal side of the placenta have been passaged in serum-free mediu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com