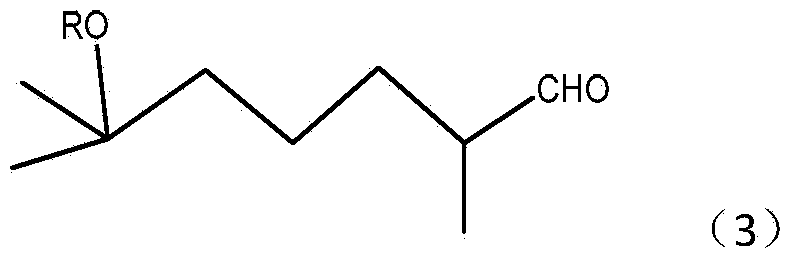
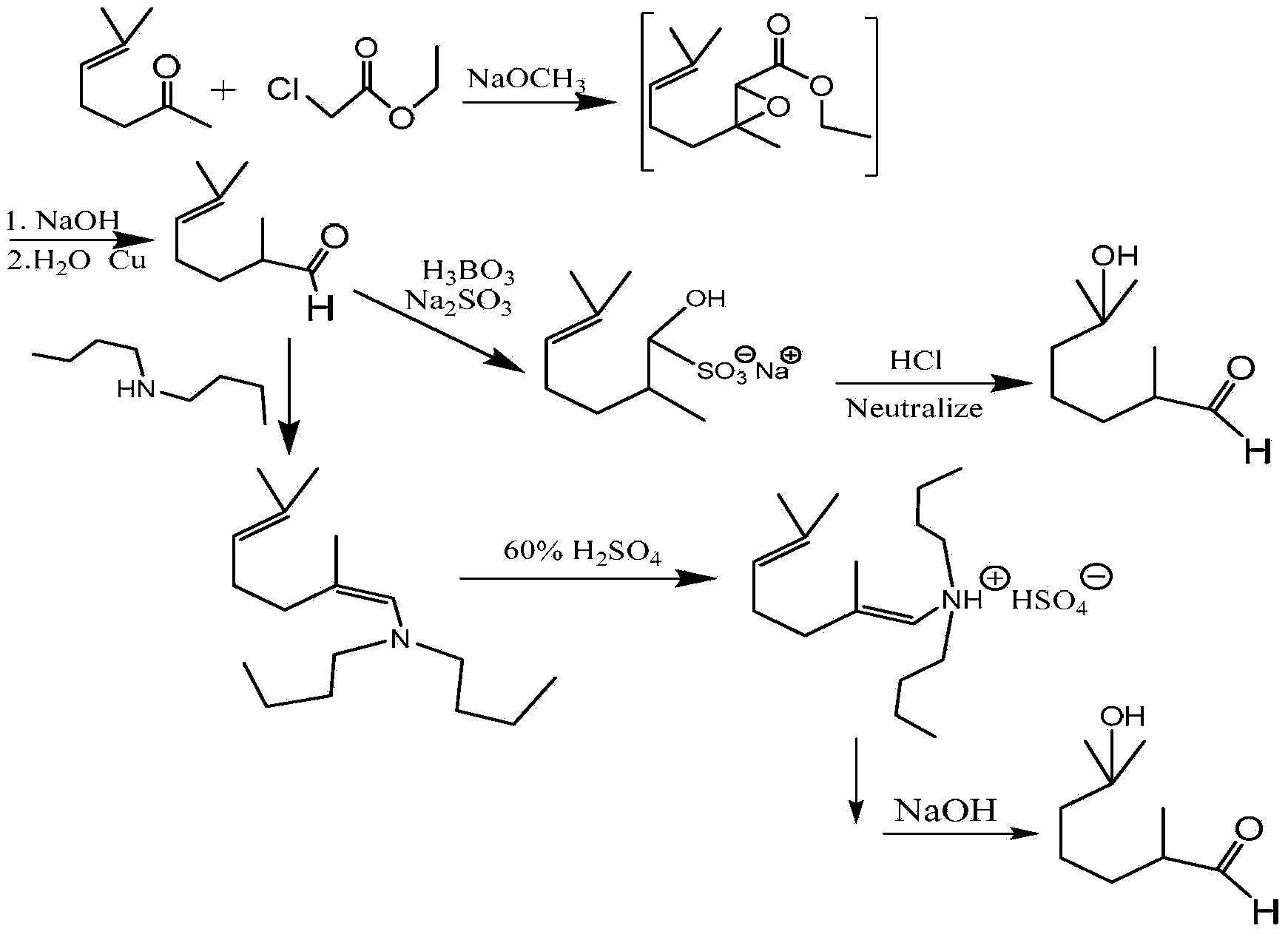
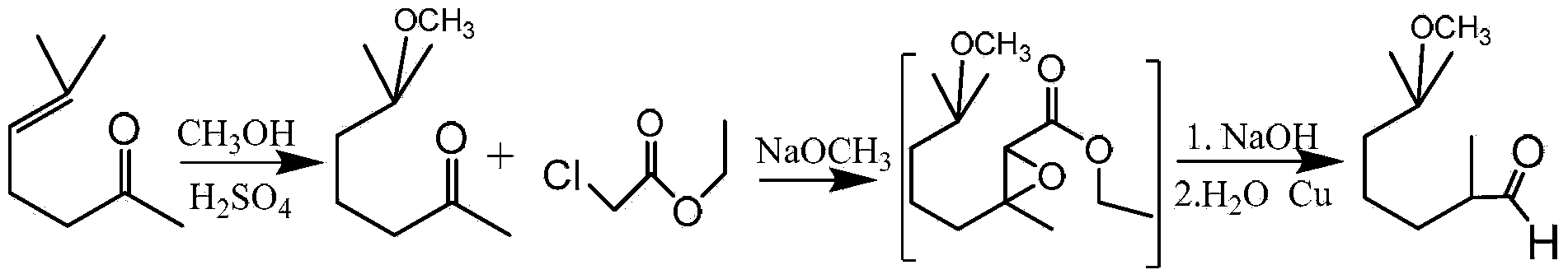
Preparation method of 2,6-dimethyl-6-alkyloxy(or hydroxyl)heptaldehyde
A technology of alkoxy and dimethyl, which is applied in the field of preparation of 2,6-dimethyl-6-alkoxy (or hydroxy) heptanal, which can solve environmental pollution, high equipment requirements, cumbersome steps, etc. problems, to achieve the effect of good industrial reliability, low separation difficulty and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A preparation method of 2,6-dimethyl-6-hydroxyl-heptanal, comprising the steps of:
[0037] (1) Ozonation reaction of dihydromyrcenol (2,6-dimethyl-2-hydroxy-7-octene)
[0038] Take 22kg of dihydromyrcenol, 44kg of solvent xylene, and 44kg of water in a 150L bubble column reactor (height-to-diameter ratio 10:1), 1.5m from the bottom of the reactor 3 The speed of / h / (kg raw material) is slowly passed into O 3 The mole percentage is 10% O 3 / O 2 The reaction temperature of the mixture is 10°C. After the ozonation reaction occurs for 2 hours, use KI test paper to test the gas at the outlet of the reactor. The KI test paper turns blue. After the material is discharged from the bottom of the reactor, it is separated by a phase separator, and the oil phase is taken out. 58kg of dihydromyrcenol ozonation product is stored in a storage tank, filled with nitrogen for protection.
[0039] (2) Reduction reaction of dihydromyrcenol (2,6-dimethyl-2-hydroxy-7-octene) ozonation pr...
Embodiment 2
[0042] A preparation method of 2,6-dimethyl-6-methoxy-heptanal, comprising the steps of:
[0043] (1) Ozonation reaction of dihydromyrcene methyl ether (2,6-dimethyl-2-methoxy-7-octene)
[0044] Take 260g of dihydromyrcene methyl ether, 130g of solvent acetonitrile, and 260g of water in a 1L bubble column reactor (height-to-diameter ratio 10:1). 3 The speed of / h / (kg raw material) is slowly passed into O 3 The mole percentage is 8% O 3 / O 2 The reaction temperature of the mixture is 12°C. After the ozonation reaction occurs for 3.5 hours, use the KI test paper to test the gas at the gas outlet of the reactor. The KI test paper turns blue, and continue to pass O 3 / O 2 After the mixture was reacted for 1 hour, the material was transferred from the reactor to a separatory funnel, and 370 g of the ozonated product of dihydromyrcene methyl ether in the oil phase was taken out and stored in a bottle under nitrogen protection.
[0045] (2) Reduction reaction of dihydromyrcene m...
Embodiment 3
[0048] A preparation method for 2,6-dimethyl-6-acetoxy-heptanal, comprising the steps of:
[0049] (1) Ozonation reaction of dihydromyrcenyl acetate (2,6-dimethyl-2-acetoxy-7-octene)
[0050] Take 520g of dihydromyrcenyl acetate, 260g of acetonitrile as a solvent, and 520g of water in a 2L bubble column reactor (height-to-diameter ratio 8:1). 3 The speed of / h / (kg raw material) is slowly passed into O 3 The molar percentage is 7% O 3 / O 2 The reaction temperature of the mixture is 11°C. After the ozonation reaction occurs for 4 hours, use the KI test paper to test the gas at the outlet of the reactor. The KI test paper turns blue, and continue to pass O 3 / O 2 After the mixture was reacted for 1 h, the material was transferred from the reactor to a separatory funnel, and 740 g of the ozonated product of dihydromyrcenyl acetate in the oil phase was taken out and stored in a bottle under nitrogen protection.
[0051] (2) Reduction reaction of 2,6-dimethyl-2-acetoxy-7-octene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com