One-pot synthesis of n-hydrocarbyl cyclic lactam derivatives
A technology for a hydrocarbon acyl cyclic lactam and a synthesis method, which is applied in the field of one-pot synthesis of N-hydrocarbon acyl cyclic lactam derivatives, can solve the problems of harsh reaction conditions, high reaction temperature, short production cycle and the like, and achieves easy reaction, Simple operation, short process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation of 6-octylamidohexyl hydroxamic acid
[0055] Control the temperature of the reactor at 30°C, add 11.32 parts of caprolactam with a purity of 99% and 19.44 parts of octanoyl chloride with a purity of 99% into the reactor, add 22.64 parts of dichloromethane as a solvent, and divide 10.08 parts of sodium bicarbonate under stirring Multiple batches were added to the reactor, and after 3 hours of reaction; then 6.95 parts of hydroxylamine hydrochloride and 21.20 parts of sodium carbonate were added to the above-mentioned reactor, and 23.7 parts of methanol were added as a solvent for a further 3 hours of reaction to obtain the required 6-n- The product of decanoylaminohexyl hydroxamate is adjusted to a pH value of about 5 with hydrochloric acid solution to obtain 6-n-decylamidohexylhydroxamic acid. Analysis showed that the purity of 6-n-octylamidohexyl hydroxamic acid was 87.3%, and the yield was 90.4%. Product Mr: 272.21, detected by LC-MS MS: 273.1 ( Figure...
Embodiment 2
[0057] Preparation of 6-benzamidohexyl hydroxamic acid
[0058] Control the temperature of the reactor at 25°C, add 11.32 parts of caprolactam with a purity of 99% and 16.80 parts of benzoyl chloride with a purity of 99% into the reactor, add 45 parts of dichloromethane as a solvent, and divide 6.8 parts of calcium oxide under stirring Add multiple batches to the reactor, and react for 1 hour; then add 6.95 parts of hydroxylamine hydrochloride and 21.20 parts of sodium carbonate to the above reactor, and add 23.7 parts of methanol as a solvent, and react for a further 2 hours to obtain 6-benzamide Base hexyl hydroxamate product, adjust the pH value to about 5 with nitric acid solution to obtain 6-benzamidohexyl hydroxamic acid. Analysis showed that the purity of 6-benzamidohexyl hydroxamic acid was 86.4%, and the yield was 90.2%. Product Mr: 250.13, detected by LC-MS MS: 249.9 ( Figure 6 ).
Embodiment 3
[0060] Preparation of 6-n-Dodecylamidohexyl Hydroxamic Acid
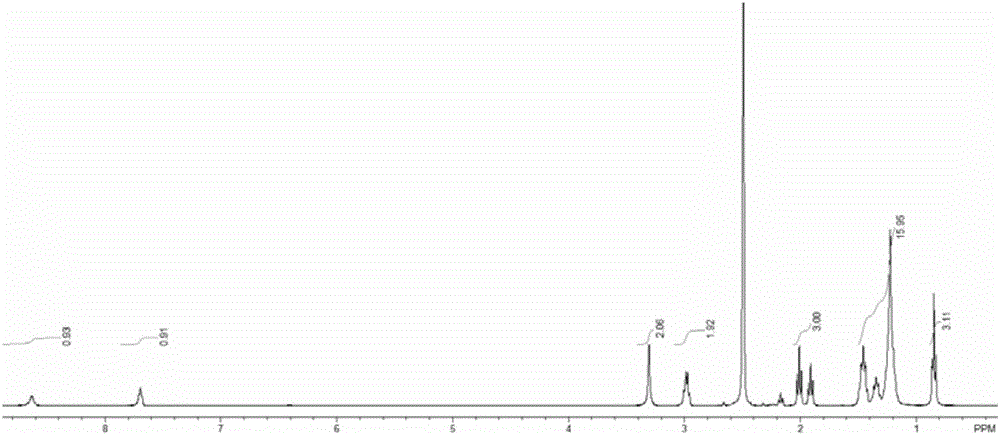
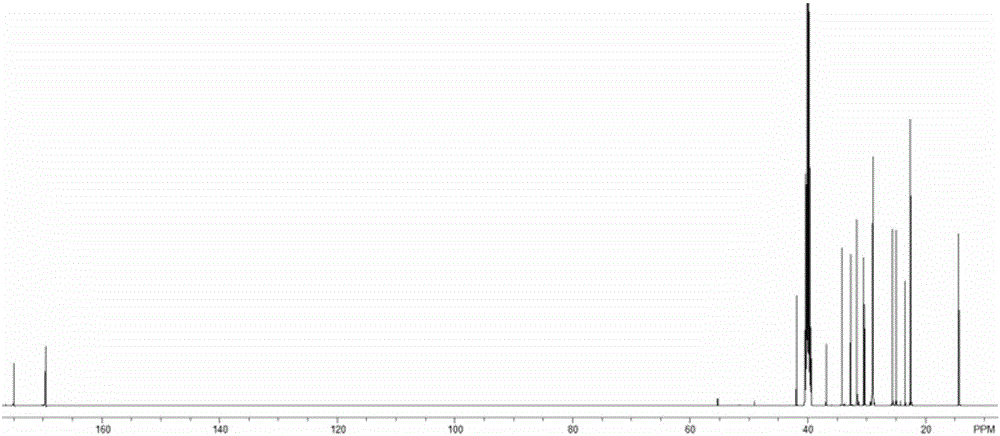
[0061] The temperature of the reactor in Example 1 was adjusted to 15° C., 19.44 parts of octanoyl chloride was changed to 26.17 parts of dodecanoyl chloride, and other conditions remained unchanged, and the obtained 6-n-dodecanoylhexyl hydroxamic acid product was obtained. Analysis showed a purity of 85.4% and a yield of 88.2%. Product Mr: 328.21, detected by mass spectrometry MS: 329.2 ( Figure 7 ), the product is 1 HNMR ( image 3 )and 13 CNMR ( Figure 4 ) was confirmed as the structure of the desired product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com