A gold nano-drug carrier with dual effects of overcoming and avoiding p-glycoprotein-mediated tumor multidrug resistance
A multi-drug resistance and P-glycoprotein technology, applied in the direction of anti-tumor drugs, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
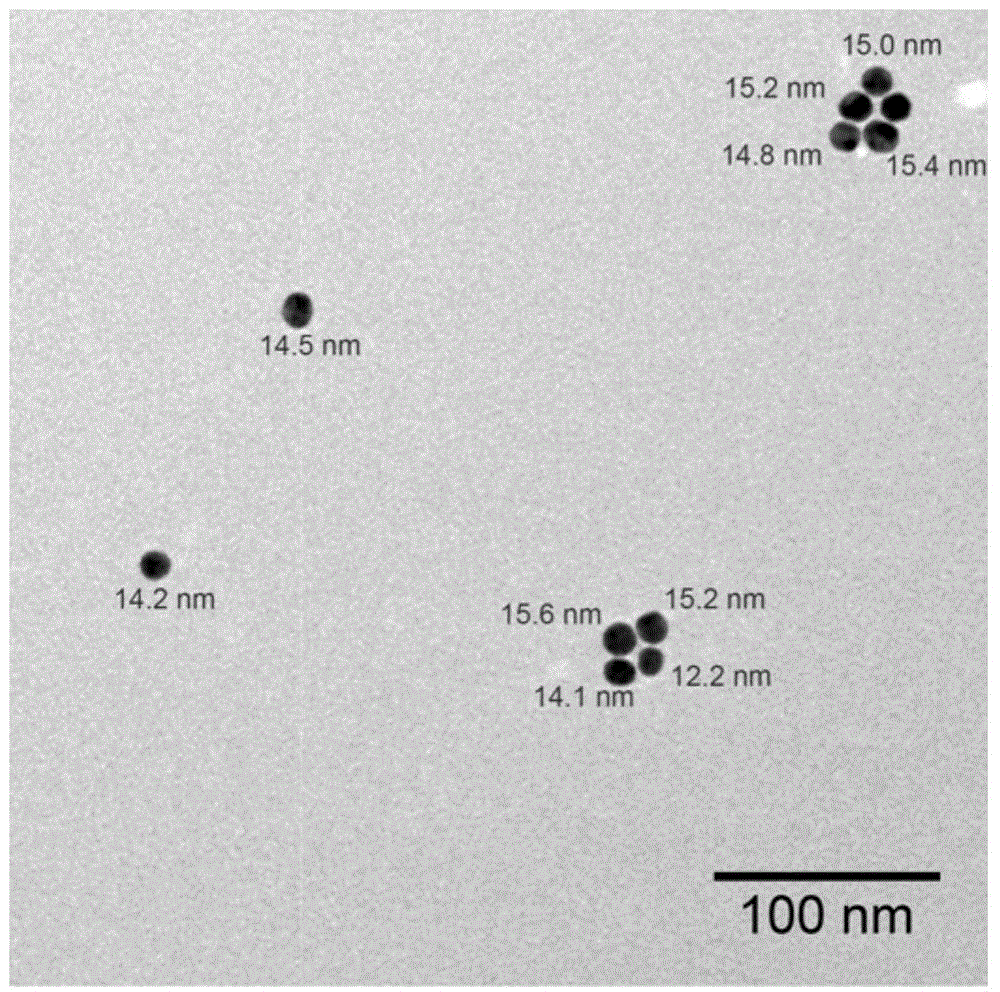
[0062] Preparation of 15nm gold nanomaterials:
[0063] 104.56 mg (0.25 mmol) HAuCl 4 4H 2 0 dissolved in 300mL PALL water, heated to boiling. Dissolve 264mg (0.90mmol) of sodium citrate in 30mL of PALL water, preheat to 80°C and quickly add to the above boiling solution. The color of the solution quickly changed to blue-purple and finally wine red. In boiling state, continue to stir for 10 min, then cool to room temperature naturally. by and (0.22μm, Millipore) filter, adjust the pH to 8.0 with 0.5M NaOH solution, measure the gold element content by inductively coupled plasma mass spectrometry (ICP-MS) and adjust the concentration of nanomaterials to 5nM, set aside.
Embodiment 2
[0065] Preparation of drug-loaded ligand (CD-SH):
[0066]
[0067] Preparation of β-cyclotine sulfonate (CD-OTs): In an ice bath, 3.0 g (1.6 mmol) of β-cyclotine was dispersed in 25 mL PALL water, and 8.2 M NaOH solution was added dropwise under stirring, followed by With the addition of NaOH, cyclodextrin gradually dissolved in water. Afterwards, 1 mL of acetonitrile solution containing 437 mg (2.3 mmol) p-toluenesulfonyl chloride was added dropwise, and a white precipitate was formed immediately. After stirring for 4 hours at room temperature, the pH of the reaction system was adjusted to 6 with 3M hydrochloric acid. The white precipitate was obtained by filtration, and recrystallized twice to finally obtain β-cycloquine sulfonate (CD-OTs, 1.1 g, yield 53.3%). HR-MS (m / z): 1289.3796 (MH + ).
[0068] Preparation of amino β-cyclotine (CD-EDA): Dissolve 1.0g of β-cyclotine sulfonate (CD-OTs, 0.8mmol) in 4mL of ethylenediamine, stir at 70°C for 4h under nitrogen protecti...
Embodiment 3
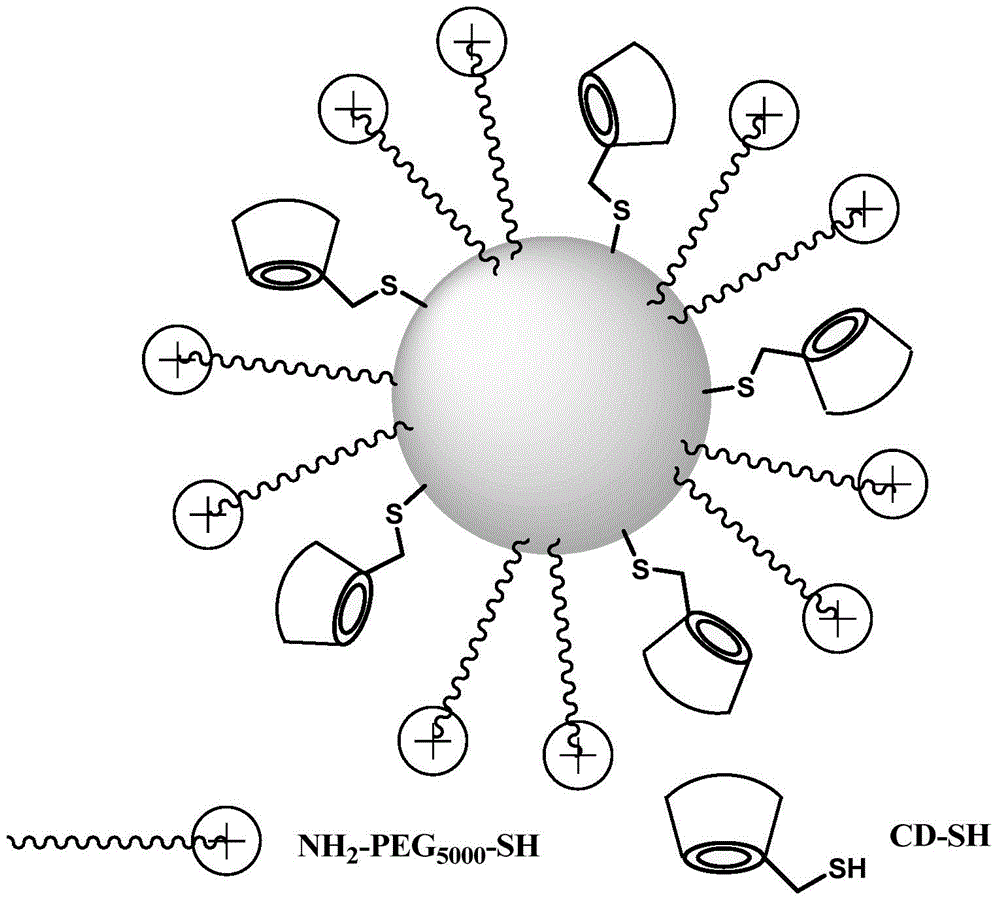
[0071] Preparation of novel gold nano drug carrier:
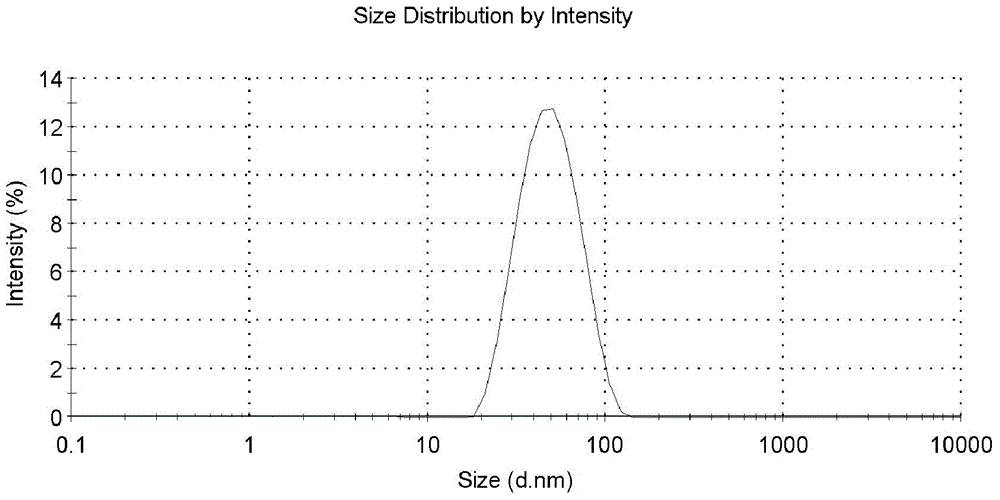
[0072] synergistic ligand NH 2 -PEG5000-SH6mg (1.2mmol) was added to 200mL of 15nm gold nano stock solution under stirring, and stirred vigorously at 4°C for 24h. With stirring, 50 μL of the PBS solution of the drug-loaded ligand CD-SH was added to the above solution, and the stirring was continued at 4° C. for 24 h. The reaction mixture was placed in a 10KD centrifugal filter, and centrifuged at 5000g for 30 minutes to separate and remove excess ligand; the separated solid was dissolved in deionized water, and after conventional ultrasonic treatment, centrifuged again at 5000g for 30± 5 minutes, and repeated centrifugation and washing 6 times (PALL water, 40mL×6). After the last washing step, the gold nanocarriers were dissolved in 5mL high-purity water and used as the original solution. After digestion, the gold element was determined by ICP-MS method content, and adjust the concentration of gold nano drug carrier to 100n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com