Preparation method and application of acid sensitive doxorubicin prodrug based on polyethylene glycol
A polyethylene glycol and sensitive technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. Side effects and other issues, to achieve good water solubility and storage stability, improve blood circulation time, easy to decompose effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Polyethylene glycol (NC- a -PEG 45 - a -NC) synthesis
[0051] First, polyethylene glycol (N 3 - a -PEG 45 - a -N 3 ), p-nitrophenyl propargyl carbonate, under the action of "click" chemical reaction catalyst and ligand, a click chemical reaction occurs to prepare polyethylene glycol with a p-nitrophenyl end (NC- a -PEG 45 - a -NC). The specific synthesis method is as follows:
[0052] Dry the branched round-bottomed flask with a stirrer in an oven at 120°C for at least 24 hours, then plug it with a glass stopper, connect it to an oil pump through a latex tube, and pump it to room temperature, then introduce high-purity argon gas, and vacuumize, repeat this process After filling with argon three times, quickly add polyethylene glycol N containing diazide ethyl diacetal group at the end. 3 - a -PEG 45 - a -N 3 (489 mg, 0.22 mmol), p-nitrophenyl propargyl carbonate (255 mg, 1.16 mmol), cuprous bromide (0.014 g, 0.10 mmol) and pentamethyldiethyle...
Embodiment 2
[0054] Example 2: Acid-sensitive doxorubicin prodrug based on polyethylene glycol (DOX- a -PEG 45 - a -DOX) synthesis
[0055] Dry the 50 mL round-bottomed flask and glass stopper with a stirring bar in an oven at 120 °C for at least 24 h, then cool to room temperature, add NC- a -PEG 45 - a -NC (123 mg, 0.05 mmol), doxorubicin hydrochloride (0.119 g, 0.2 mmol), triethylamine (0.1 mL, 0.6 mmol) and 10 mL dimethylformamide (DMF); After the reaction was completed, the DMF was pumped out with a vacuum oil pump at 50°C, dissolved by adding 2 mL of anhydrous methanol, dialyzed against anhydrous methanol for 24 h, followed by ultrapure water for 48 h, and freeze-dried to Constant weight, to obtain dark red solid product (DOX- a -PEG 45 - a -DOX, 78 mg), yield 60%.
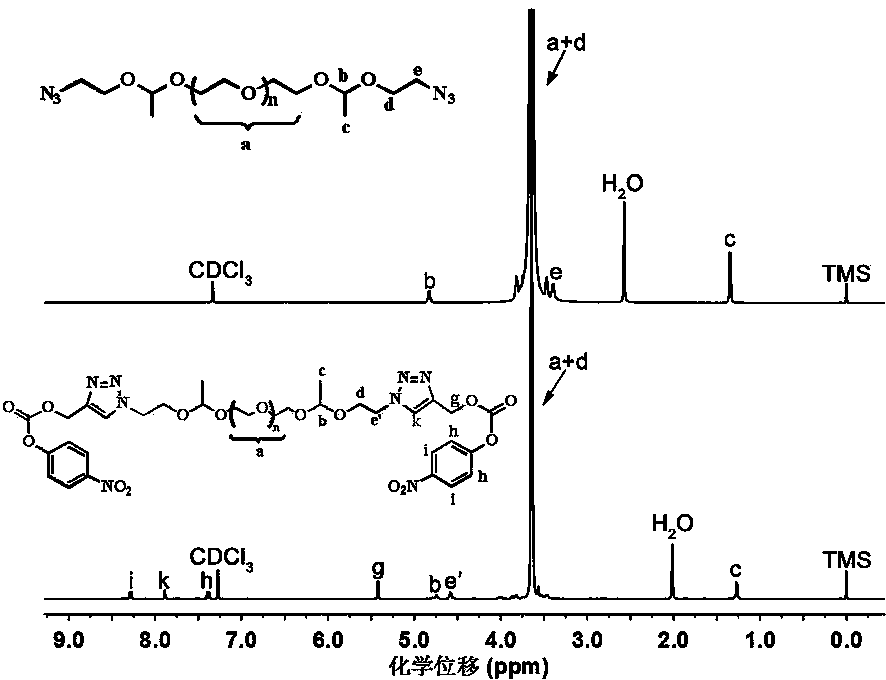
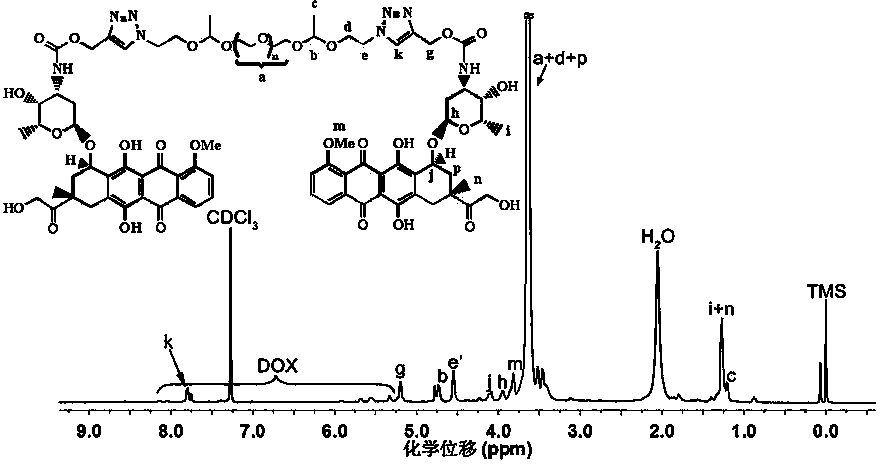
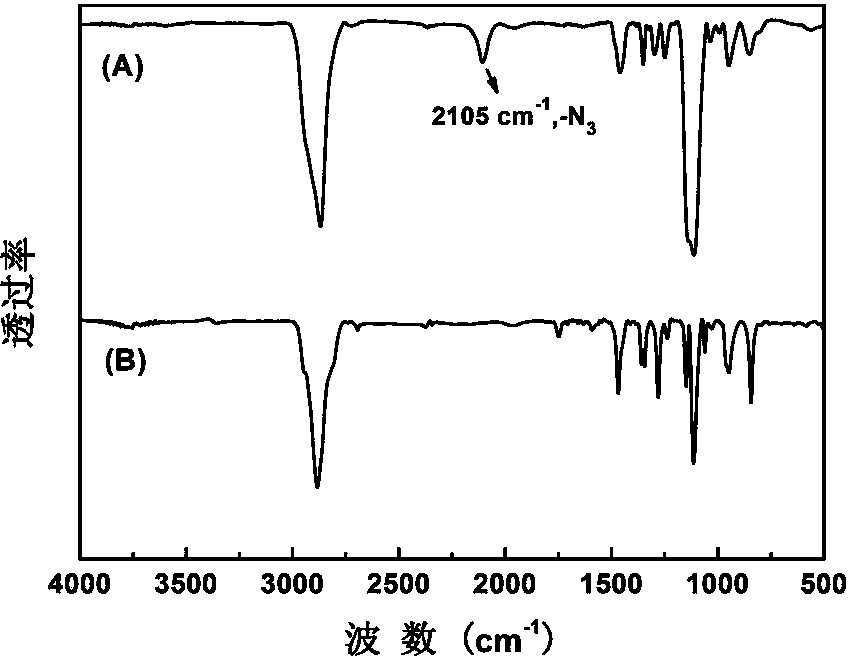
[0056] Using H NMR spectroscopy ( 1 H NMR) and infrared spectroscopy (FT-IR) verified the structure of the obtained polymer as well as the polyethylene glycol-based acid-sensitive doxorubicin prodrug. attached...
Embodiment 3
[0057] Example three: Polyethylene glycol (NC- a -PEG 90 - a -NC) synthesis
[0058] Dry the branched round-bottomed flask with a stirrer in an oven at 120°C for at least 24 hours, then plug it with a glass stopper, connect it to an oil pump through a latex tube, and pump it to room temperature, then introduce high-purity argon gas, and vacuumize, repeat this process After filling with argon three times, quickly add polyethylene glycol N containing diazide ethyl diacetal group at the end. 3 - a -PEG 90 - a -N 3 (439 mg, 0.1 mmol), p-nitrophenyl propargyl carbonate (81 mg, 0.36 mmol), cuprous bromide (0.014 g, 0.10 mmol) and pentamethyldiethylenetriamine (21 μL , 0.10 mmol), pumped with an oil pump and filled with argon three times to remove the air in the bottle, then added 20 mL of solvent tetrahydrofuran (THF) with a dry syringe, and stirred until the mixture was completely dissolved and the solution turned blue. Then the round bottom flask was moved to an oil bath at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com