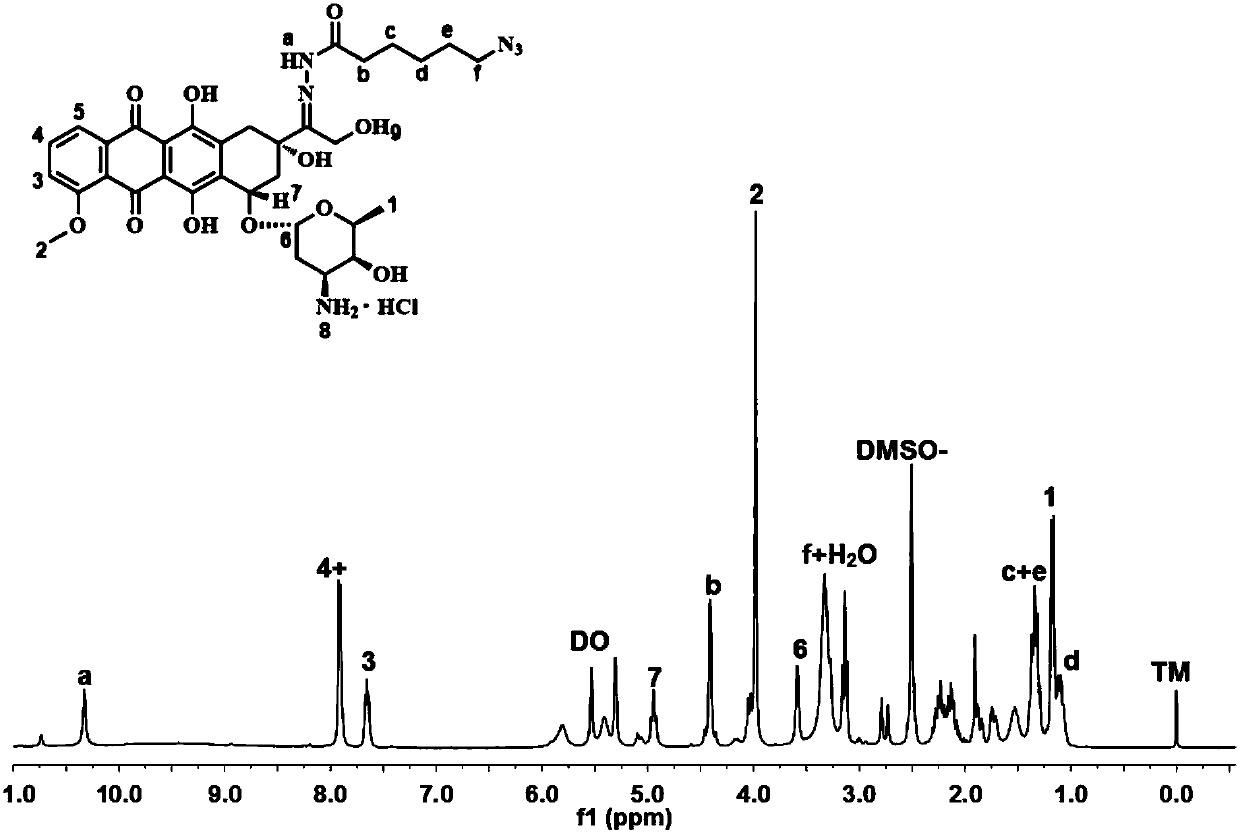
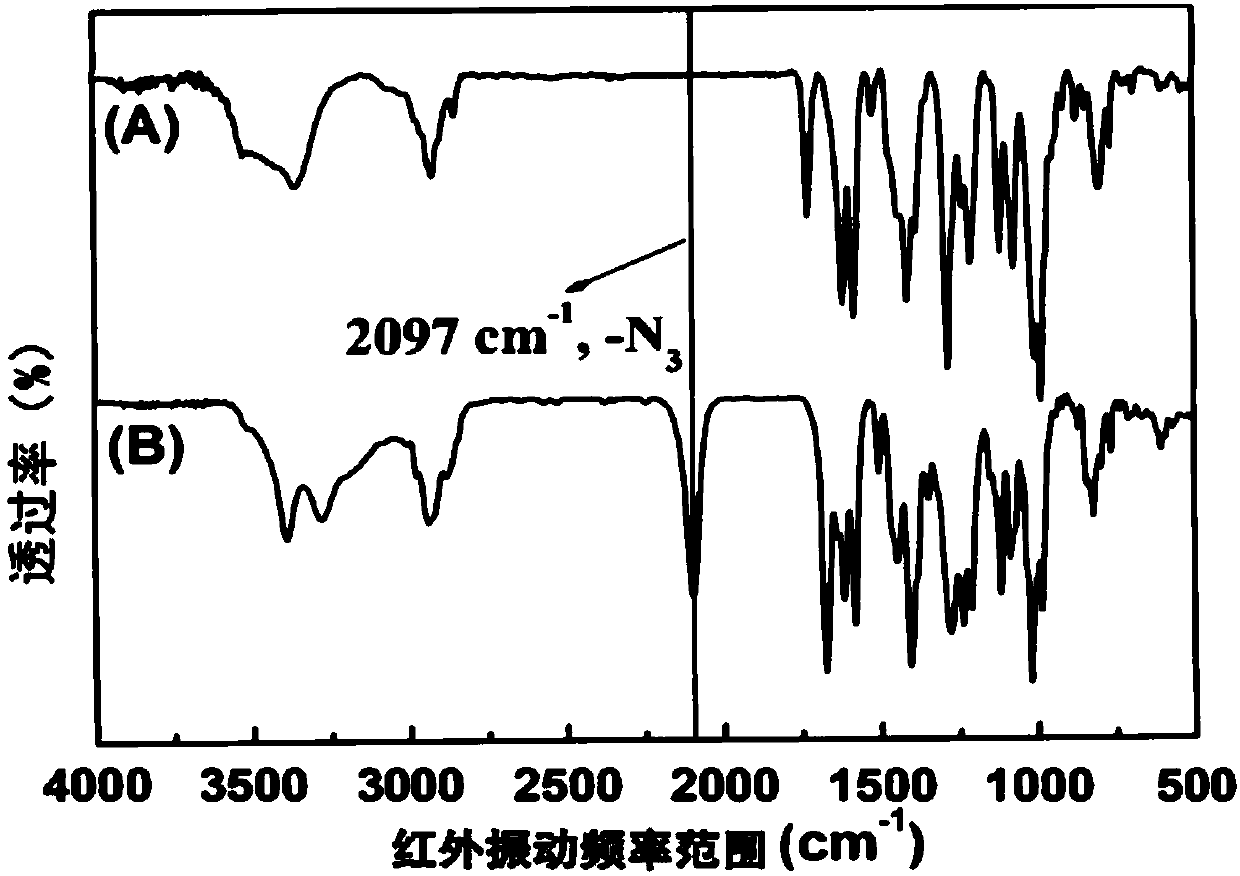
Polyphosphoester prodrug simultaneously bonding camptothecin and adriamycin amycin and preparing method and application thereof
A technology of polyphosphate and camptothecin, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., achieves the advantages of simple product purification technology, high yield, and improved blood circulation time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
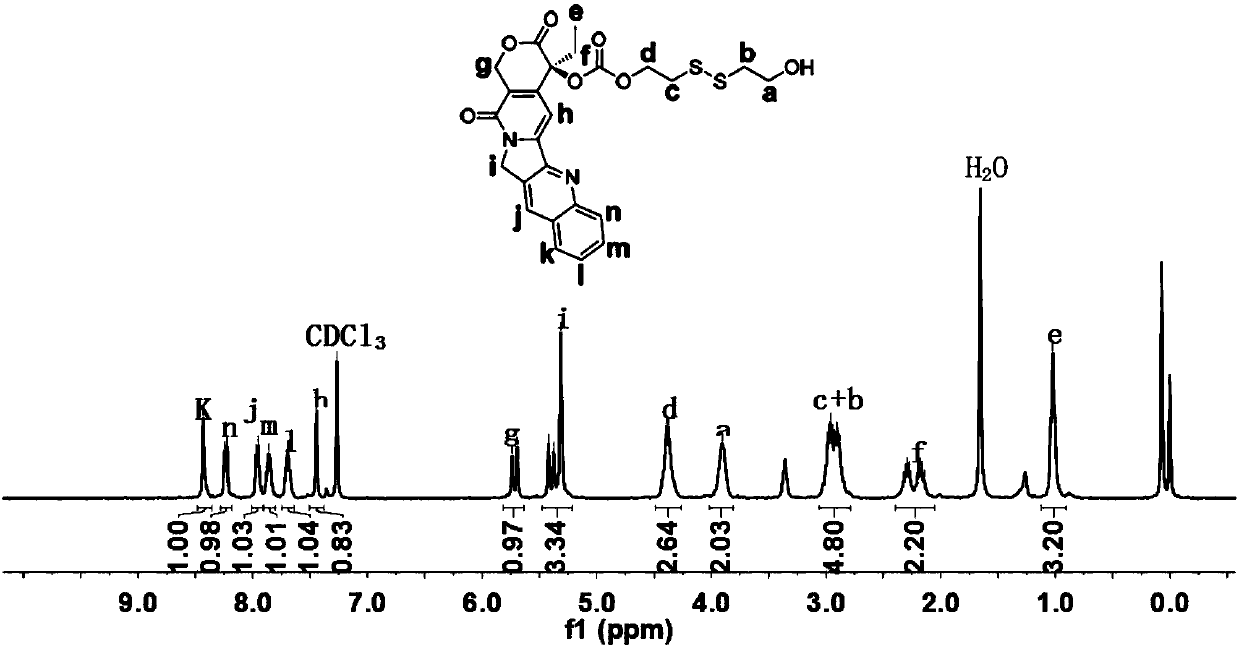
[0075] Example 1: Random copolymer CPT- ss -PBYP 30 - b -PEEP 80 Synthesis of (1:20:50)
[0076] Camptothecin dithiol compounds (CPT- ss -OH) as the initiator and stannous octoate as the catalyst, through the "one-pot method" continuous ring-opening polymerization of BYP and EOP to obtain CPT- ss -PBYP- b -PEEP random copolymer, the preparation process is carried out under anhydrous and oxygen-free conditions. The syringes and needles used in the experiment were dried in an oven at 120 °C for 5 h, and were taken out and cooled in a desiccator before the reaction. Dry the clean ampoule bottle (pre-installed with a magnetic stirring rotor) in a 120 °C oven for about 5 hours, take it out and connect it to a vacuum pump with a latex tube, fill it with high-purity argon while vacuuming, and repeat the operation three times to remove the bottle. moisture and oxygen. Add the camptothecin dithiol compound (CPT- ss-OH) (0.185 g, 0.35 mmol), BYP (1.251 g, 7.1 mmol), EOP (2.645...
Embodiment 2
[0077] Example 2: Random copolymer CPT- ss -PBYP 28 - b -PEEP 33 Synthesis
[0078] Camptothecin dithiol compounds (CPT- ss -OH) as the initiator and stannous octoate as the catalyst, through the "one-pot method" continuous ring-opening polymerization of BYP and EOP to obtain CPT- ss -PBYP- b -PEEP random copolymer. The preparation process is carried out under anhydrous and oxygen-free conditions. The syringes and needles used in the experiment were dried in an oven at 120 °C for 5 h, and were taken out and cooled in a desiccator before the reaction. Dry the clean ampoule bottle (pre-installed with a magnetic stirring rotor) in a 120 °C oven for about 5 hours, take it out and connect it to a vacuum pump with a latex tube, fill it with high-purity argon while vacuuming, and repeat the operation three times to remove the bottle. Moisture and oxygen; in the ampoule, add the camptothecin dithiol compound (CPT- ss -OH) (0.189 g, 0.35 mmol), BYP (1.249 g, 7.1 mmol), EOP (1...
Embodiment 3
[0079] Example 3: Random copolymer CPT- ss -PBYP 68 - b -PEEP 53 Synthesis
[0080] Camptothecin dithiol compounds (CPT- ss -OH) as the initiator and stannous octoate as the catalyst, through the "one-pot method" continuous ring-opening polymerization of BYP and EOP to obtain CPT- ss -PBYP- b -PEEP random copolymer. The preparation process was carried out under anhydrous and oxygen-free conditions. The syringes and needles used in the experiment were dried in an oven at 120 °C for 5 h, and were taken out and cooled in a desiccator before the reaction. Dry the clean ampoule bottle (pre-installed with a magnetic stirring rotor) in a 120 °C oven for about 5 hours, take it out and connect it to a vacuum pump with a latex tube, fill it with high-purity argon while vacuuming, and repeat the operation three times to remove the bottle. moisture and oxygen. Add the camptothecin dithiol compound (CPT- ss -OH) (0.189 g, 0.35 mmol), BYP (1.873 g, 10.5 mmol), EOP (2.623 g, 17.5 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com