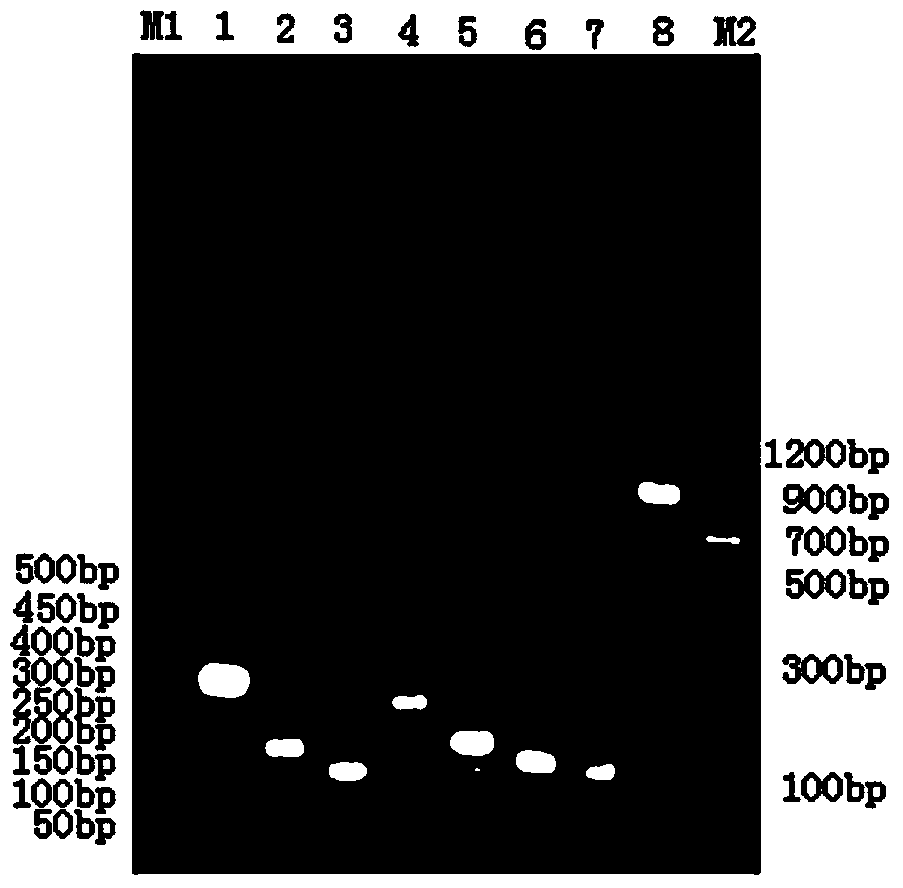
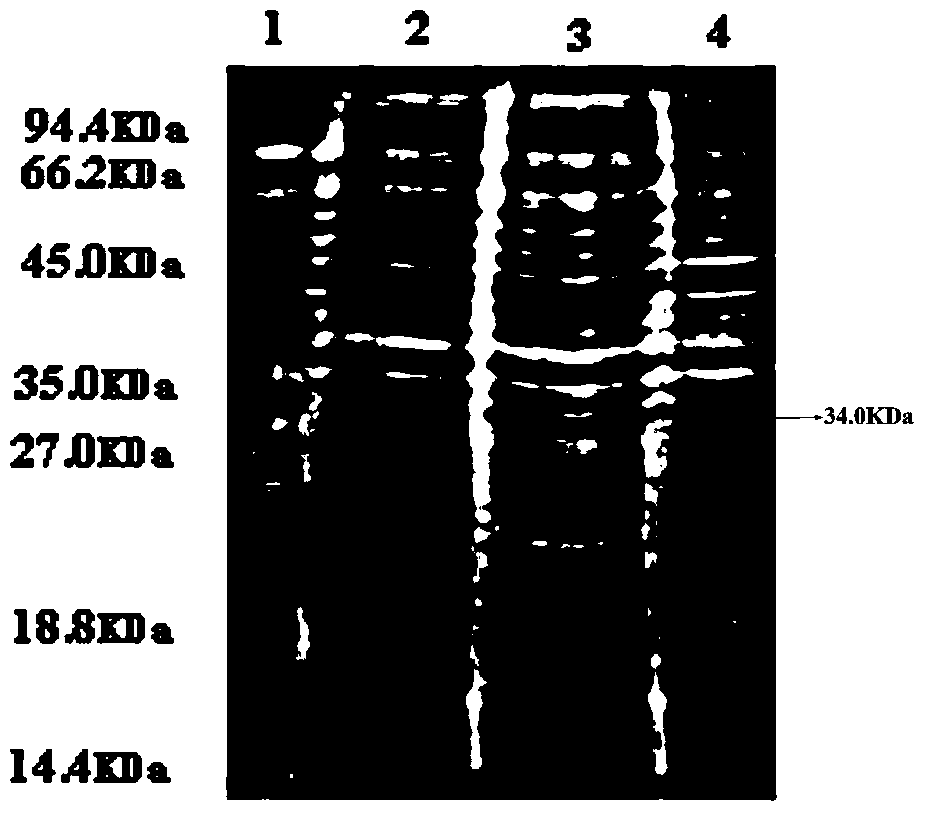
Human-derived urate oxidases with catalytic activity
A urate oxidase and catalytic activity technology, applied in the field of human urate oxidase, can solve the problems of unconfirmed immunogenicity, reduce the immunogenicity of protein drugs, prolong the half-life of protein drugs, etc., and achieve the effect of low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1 Human-pig urate oxidase chimeric protein H 1-2 P 3-8 Construction of DNA and its recombinant expression in Escherichia coli BL21
[0088] Obtain the plasmid containing the template: inoculate glycerol tubes containing Escherichia coli engineering bacteria containing human urate oxidase gene and porcine urate oxidase gene respectively, the inoculation amount is 1%, culture overnight, and extract the plasmid.
[0089] H 1-2 ,P 3-8 Fragments, and then use the method of overlap extension PCR to obtain H 1-2 P 3-8 DNA.
[0090] get H 1-2 P 3-8 DNA: The first stage of PCR: the template sequence is the above plasmid containing the human uric acid oxidase gene, the primers are primer 1 and primer 6, and the PCR conditions are: 95°C for 30s, 55°C for 30s, 72°C for 35s, a total of 30 cycles, the first One cycle of denaturation at 95°C for 5 minutes, the last cycle of extension at 72°C for 10 minutes, and finally the product H 1-2 Fragment; the second stage of P...
Embodiment 2
[0096] Example 2 Human-pig urate oxidase chimeric protein P 1-2 h 3 P 4-8 Construction of DNA and its recombinant expression in Escherichia coli BL21
[0097] The same method as in Example 1 was used to obtain plasmids containing human and porcine urate oxidase gene sequence templates.
[0098] get P 1-2 H3P 4-8 DNA: The first stage of PCR: the template sequence is pET-22b(+)-PU, the primers are primer 8 and primer 3, the PCR conditions are: 95°C for 30s, 55°C for 30s, 72°C for 35s, a total of 30 cycles, the first The first cycle was denatured at 95°C for 5 minutes, and the last cycle was extended at 72°C for 10 minutes to obtain the product P 1-2Fragment; the second stage of PCR: the template sequence is pET-22b(+)-HU, the primers are primer 7 and primer 9, the PCR conditions are: 95°C for 30s, 55°C for 30s, 72°C for 20s, a total of 30 cycles, the first The first cycle was denatured at 95°C for 5 minutes, the last cycle was extended at 72°C for 10 minutes, and finally t...
Embodiment 3
[0100] Example 3 Human-pig urate oxidase chimeric protein P 1-3 h 4 P 5-8 Construction of DNA and its recombinant expression in Escherichia coli BL21
[0101] The same method as in Example 1 was used to obtain plasmids containing human and porcine urate oxidase gene sequence templates.
[0102] get P 1-3 h 4 P 5-8 DNA: The first stage of PCR: the template sequence is pET-22b(+)-PU, the primers are primer 11 and primer 3, the PCR conditions are: 95°C for 30s, 55°C for 30s, 72°C for 45s, a total of 30 cycles, the first The first cycle was denatured at 95°C for 5 minutes, and the last cycle was extended at 72°C for 10 minutes to obtain the product P 1-3 Fragment; the second stage of PCR: the template sequence is pET-22b(+)-HU, the primers are primer 12 and primer 13, the PCR conditions are: 95°C for 30s, 55°C for 30s, 72°C for 15s, a total of 30 cycles, the first The first cycle is denatured at 95°C for 5 minutes, and the last cycle is extended at 72°C for 10 minutes to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com