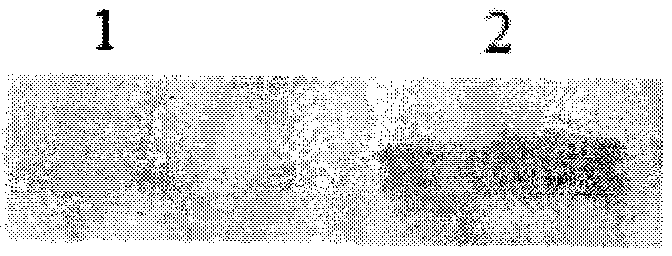Method for preparing recombinant bovine enterokinase catalytic subunit
A technology of enterokinase catalytic subunit and kinase catalytic subunit, which can be applied in the field of genetic engineering and can solve the problems of low expression level and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
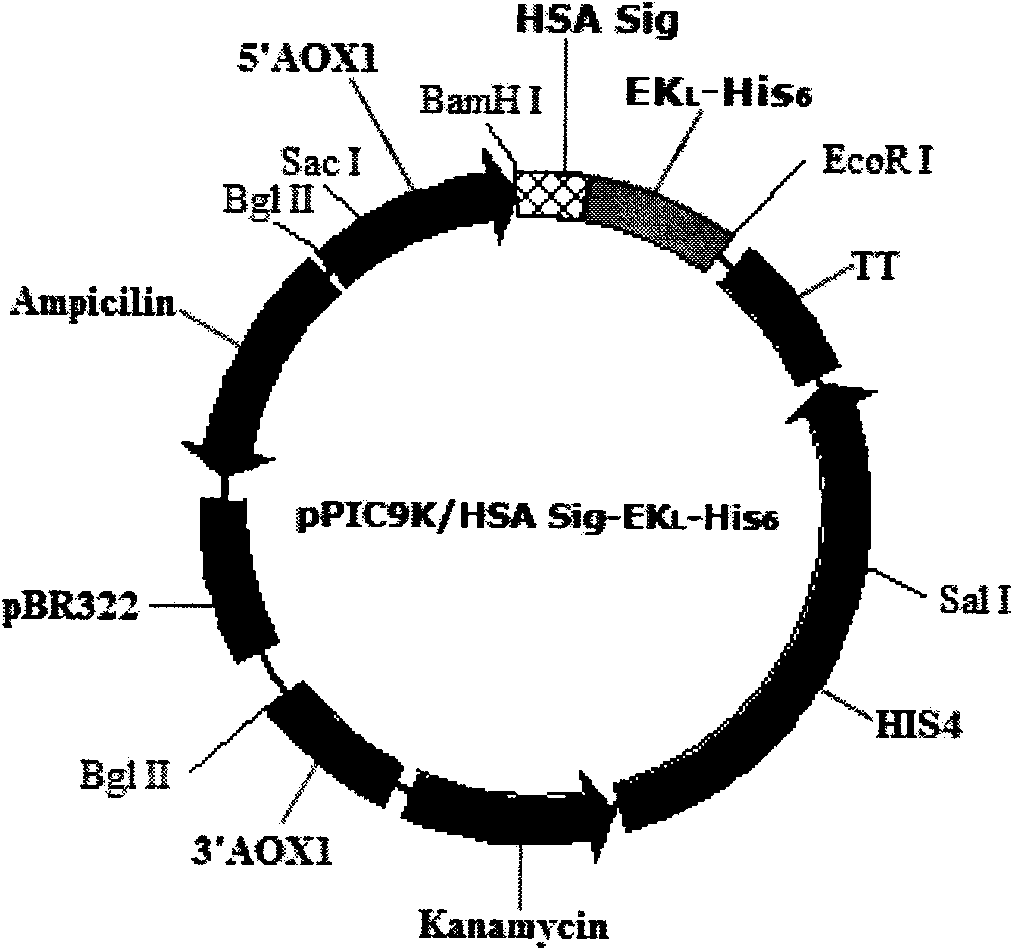
[0032] Example 1 Human albumin signal peptide-bovine enterokinase catalytic subunit BEK L Fusion gene design and cloning
[0033] According to the amino acid sequence of human albumin signal peptide and the amino acid sequence of bovine enterokinase catalytic subunit, the codon optimization design software OPTIMIZER( http: / / genomes.urv.es / OPTIMIZER ) and Gene Designer ( http: / / www.DNA20.com ) designed the human albumin signal peptide-bovine enterokinase catalytic subunit fusion gene sequence expressed in Pichia pastoris (see attached figure 1 ). In addition, BamHI and EcoRI restriction sites were added to the 5' and 3' ends of the gene for gene cloning, and 6×His was added to the carboxyl end of the catalytic subunit of bovine enterokinase to facilitate the separation and purification of expression products. expression label. attached figure 1 The middle shaded part is the signal peptide sequence of human serum albumin, and the position indicated by the arrow is the sig...
Embodiment 2
[0035] Example 2 Recombinant Expression Plasmid Electrotransformation and Positive Recombinant Screening and Identification
[0036] The recombinant plasmid pPIC9K / HSA signal-EK was transformed with SacI enzyme L -His 6 Enzyme digestion and linearization were performed, and then the Pichia.pastoris host strain GS115 was transformed by electroporation (electroporation parameters: 1.5KV, 25μF and 200Ω, 4ms). Immediately after electroporation, add 1 mL of pre-cooled 1M sorbitol to the electroporation cup, incubate at 30°C for 2 hours, take a certain amount (200 μL) and spread it on the MD plate, and incubate at 30°C for 2-6 days.
[0037] G418 resistance screening, after the clone grows out, wash down the single colony with 2-4ml YPD liquid medium, and measure the OD 600nm , 1OD 600nm Equivalent to 5×10 cells 7 cells / ml, and then diluted with YPD liquid medium to the order of 10 cells per 200 μl 5 , coated on YPD plates containing different concentrations of G418, the concen...
Embodiment 3
[0039] Example 3 Secretion and expression of bovine EK by Pichia pastoris L -His 6 Shake flask screening and product identification
[0040] Pick a single colony of positive clones into 50ml YPD liquid medium, 30°C, 250rpm shaking culture for 16-18 hours, transfer to 50ml BMGY liquid medium at 4% according to the seed amount, 30°C, 250rpm shaking culture until OD 600nm 2-6, 5000rpm, 5min centrifugation to harvest the bacterial cells, transfer to 30ml BMMY liquid medium to induce expression. Methanol was supplemented every 24 hours to a final concentration of 1%, and the expression was continuously induced for 72 hours. The supernatant of the fermentation broth was analyzed by SDS-PAGE to screen high-expression strains. At the same time, the anti-histidine tag 6×His tag antibody was used as the primary antibody for Western-Blot identification ( image 3 ), the results showed that the target protein could specifically react with the antibody against the histidine tag, indica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com