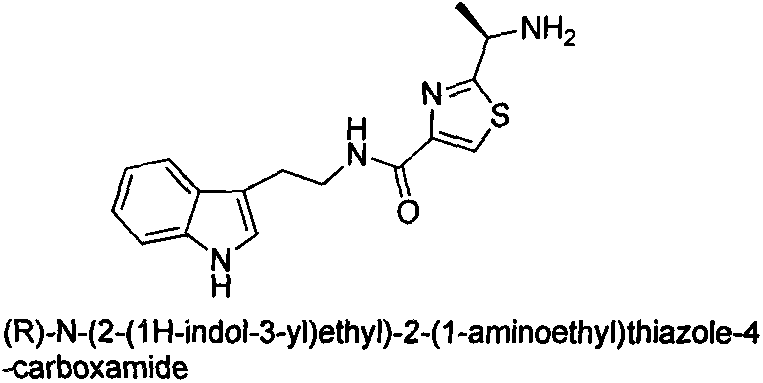
Salifying method of 2-(1-amido-ethyl)-N-phenyl thiazole-4-methanamide compound and application
A technology of phenylthiazole and compound is applied in the field of 2-(1-amino-ethyl)-N-phenylthiazole-4-carboxamide compound salt formation and application, and achieves low non-target biological toxicity and synthesis method simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
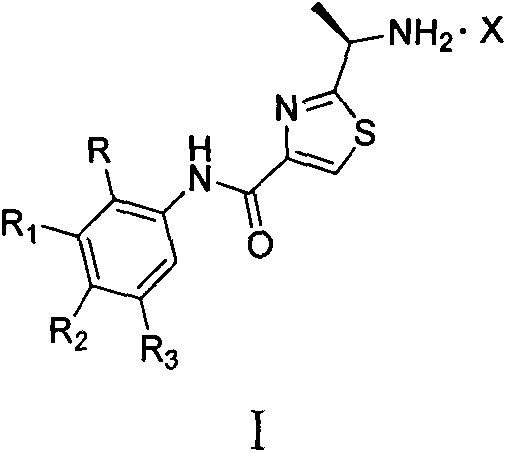
[0030] The systematic name of compound M-2 (2-(1-amino-ethyl)-N-(4-bromo-phenyl)-thiazole-4-carboxamide (English: 2-(1-aminoethyl)-N- (4-bromophenyl)thiazole-4-carboxamide, and make it into hydrochloride as follows:
[0031]
[0032] The preparation method of S-1 into hydrochloride: Accurately weigh 1 mmol of the series of derivatives in a 50ml round bottom flask and add 25ml of absolute ethanol to fully dissolve, and build a hydrochloric acid generating device: weigh about 30g of anhydrous NaCl and place it in a three-necked flask In, and gradually add concentrated sulfuric acid to it dropwise, after hydrochloric acid gas is gradually generated, the hydrochloric acid gas is dried and then introduced into the ethanol solution, and crystals are gradually precipitated until saturation. The solvent was spin-dried to obtain a solid.
[0033] When R1-R4 are different substituents, the series of hydrochlorides S-1~S-5 synthesized are as follows
[0034] Table 3 several derivati...
Embodiment 2
[0040]
[0041] Accurately weigh 1 mmol of the series of derivatives in a 50 ml round bottom flask and add 25 ml of absolute ethanol to fully dissolve it to build a hydrochloric acid generating device: add 1.2 mmol of oxalic acid to it, and gradually crystallize until it is saturated. The solvent was spin-dried to obtain a solid.
[0042] Table 5X is the derivatives of different organic acid salts
[0043]
[0044]
[0045] The series of salts Y-1 to Y-8 synthesized when X is substituted with different organic acid salts, the biological activity assay data on Scenedesmus obliques, Chlorella pyrenoidosa, and Microcystis aeruginosa are as follows:
[0046] The biological activity testing method is the same as that of the above-mentioned M series derivatives, and will not be described in detail here.
[0047] Table 6 The activity determination EC of different organic acid salt compounds on three kinds of algae 50 (mg / L)
[0048]
[0049] The experimental results sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com