Preparation of graphene oxide adsorption material modified by dendritic polymer
An adsorption material and dendritic technology, which is applied in the field of adsorbent preparation, can solve the problem of reducing the possibility of renewable circulation, and achieve the effect of strong adsorption capacity and large application space.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] First, dissolve graphene oxide in ethanol at room temperature, mix it evenly by ultrasonication for 1 hour, add it to a 500ml three-necked flask, and then add a certain amount of ethanol-dissolved dendrimer. The mass ratio of the reactant is graphene oxide : dendrimer: ethanol = 1:3:120, in the process of adding dendrimer, it can be found that the two react violently, indicating that the reaction is in progress, and the 2.0 generation polyamidoamine dissolves quickly. The whole reaction was continuously mechanically stirred in a water bath at 80°C. After the reaction was carried out for 24 hours, the resulting substance was filtered under reduced pressure and washed with ethanol three times. After drying at 80°C for 12 hours, the compound having both carboxyl and amino groups was obtained. Dendritic polymer modified graphene oxide adsorption material. In order to ensure the completeness of the reaction and improve the ability of the adsorbent to adsorb heavy metal ions,...
experiment example 2
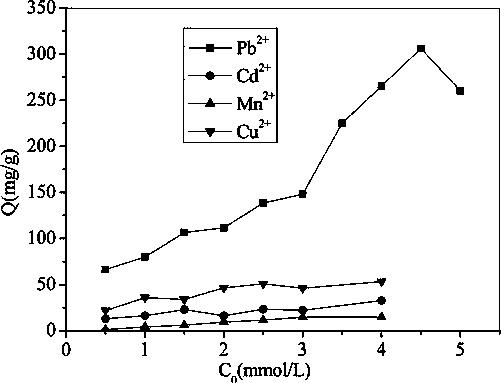
[0021] Weigh 0.1g of the adsorbent prepared in Experimental Example 1 and place it in a 250ml dry round-bottomed flask, add 100ml of the initial concentration to be pH=4.5, concentration C 0 =0.5,1,1.5,2.0,2.5,3.0,3.5,4,4.5 and 5mmol / L of Pb(NO 3 ) 2 solution, ultrasonically oscillate for 1 hour to make the adsorbent well dispersed in the solution, then place it in a constant temperature water bath at 25°C, seal the round bottom flask, and stir at a constant temperature. After 3 hours, take the adsorbed solution and filter it with a funnel. A photometer measures the concentration of lead ions remaining in the filtrate, and then calculates the adsorption capacity of the adsorbent according to equation (1).
[0022] The experimental results show that: at a temperature of 25°C and a pH of 4.5, the adsorbent prepared in Experimental Example 1 has an adsorption capacity of 306.09 mg / g of lead ions.
experiment example 3
[0024] Weigh 0.1g of the adsorbent prepared in Experimental Example 1 and place it in a 250ml dry round-bottomed flask, add 100ml of the Cd(NO 3 ) 2 solution, ultrasonically oscillate for 1 hour to make the adsorbent well dispersed in the solution, then place it in a constant temperature water bath at 25°C, seal the round bottom flask, and stir at a constant temperature. After 3 hours, take the adsorbed solution and filter it with a funnel. The photometer measures the concentration of cadmium ions remaining in the filtrate, and then calculates the adsorption amount of the adsorbent according to (1).
[0025]The experimental results show that: at 25°C, the adsorption capacity of cadmium ions at pH=2.0, 2.5, 3.0, 3.5, 4.0, 4.5 and 5.0 is 2.3~42.24mg / g. The adsorbent prepared in the paper has the largest adsorption capacity of cadmium ion, which is 42.24mg / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com