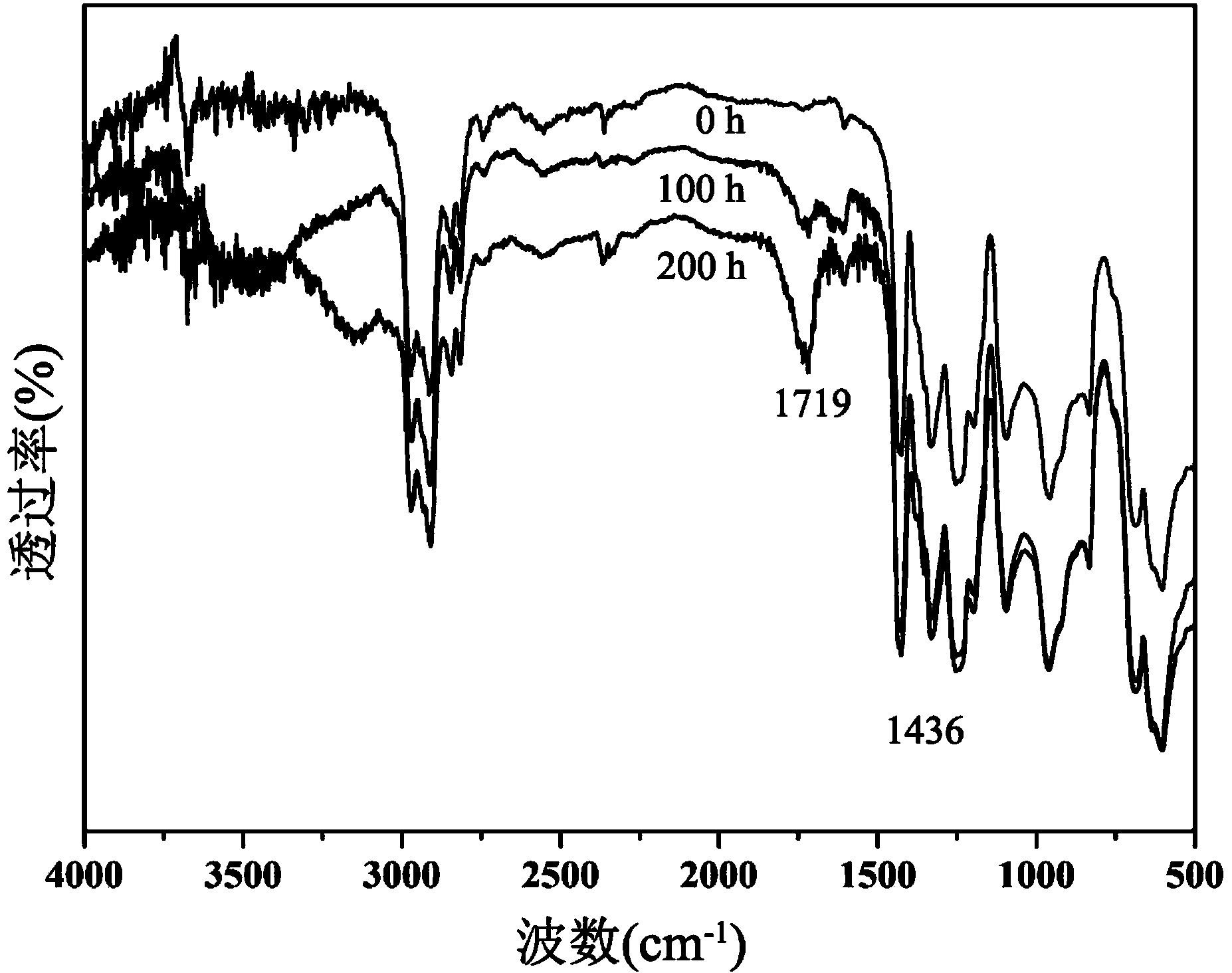
Ultraviolet light stabilizer based on polyvinyl chloride and preparation method thereof
A technology of ultraviolet light and stabilizer, which is applied in the field of polyvinyl chloride-based ultraviolet light stabilizer and its preparation, can solve the problems of non-uniform dispersion, decreased mechanical properties of polymers, and easy volatilization, etc., and achieves easy large-scale industrial production, The effect of avoiding environmental pollution problems and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0083] The preparation method of ultraviolet stabilizer of the present invention comprises the following steps:
[0084]
[0085] (i) providing a compound of formula 1 and a compound of formula 3, wherein,
[0086] Polyvinyl chloride and sodium azide react under the effect of a phase transfer catalyst to obtain a compound of formula 1,
[0087] Formula 2 compound and 2-bromopropyne reflux reaction under the effect of alkali to obtain formula 3 compound;
[0088]
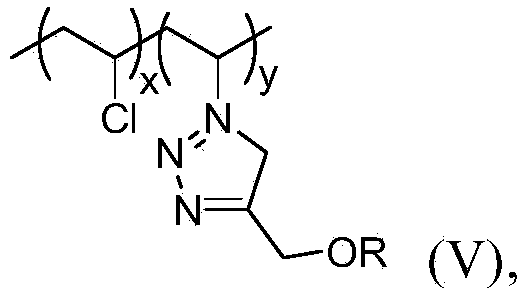
[0089] (ii) the compound of formula 1 reacts with the compound of formula 3 to obtain the UV light stabilizer shown in formula V,
[0090] Among them, n=x+y;
[0091] x, y and R are defined as previously described.
[0092] The phase transfer catalyst is selected from the group consisting of tetrabutylammonium bromide, tetra-n-octylammonium bromide and benzyltriethylammonium chloride.
[0093] In another preferred example, the molar ratio of polyvinyl chloride, sodium azide and phase transfer catalyst is 1:...
Embodiment 1
[0125] Synthesis of azide-substituted polyvinyl chloride (compound of formula 1)
[0126]
[0127] Add polyvinyl chloride (3g, 0.14mmol, number average molecular weight is 22000g / mol), sodium azide (3.8g, 58.46mmol), tetrabutylammonium bromide (0.77g, 2.38mmol) and 30mL THF was reacted at 50°C for different times, as shown in Table 1.
[0128] After stopping the reaction, precipitate in methanol and dry to obtain white polyvinyl chloride powders with different azide molar substitution ratios.
[0129] 1 H NMR, (ppm): 2.0, 4.0, 4.5.
[0130] The results of the azide molar substitution ratio y / n at different reaction times are shown in Table 1.
[0131] The azide molar substitution ratio of different reaction times in table 1 y The result of / n
[0132] Compound No. of Product Formula 1
[0133] *Note: Product number 1-1 refers to the compound of formula 1 corresponding to the reaction time of 6 hours, and so on.
Embodiment 2
[0135] Synthesis of Alkyne Substituted Ultraviolet Light Antiseptic (Compound of Formula 3)
[0136]
[0137] Add ultraviolet absorber (compound of formula 2) (10mmol), potassium carbonate (2.8g, 20mmol), 3-bromopropyne (0.8mL, 11mmol) and 25mL acetone into the reaction flask, and reflux for 24 hours. Stop the reaction, and perform flash column chromatography to obtain an alkynyl-substituted ultraviolet absorber (compound of formula 3). The reaction results are shown in Table 2.
[0138] Table 2 embodiment 2 reaction result
[0139]
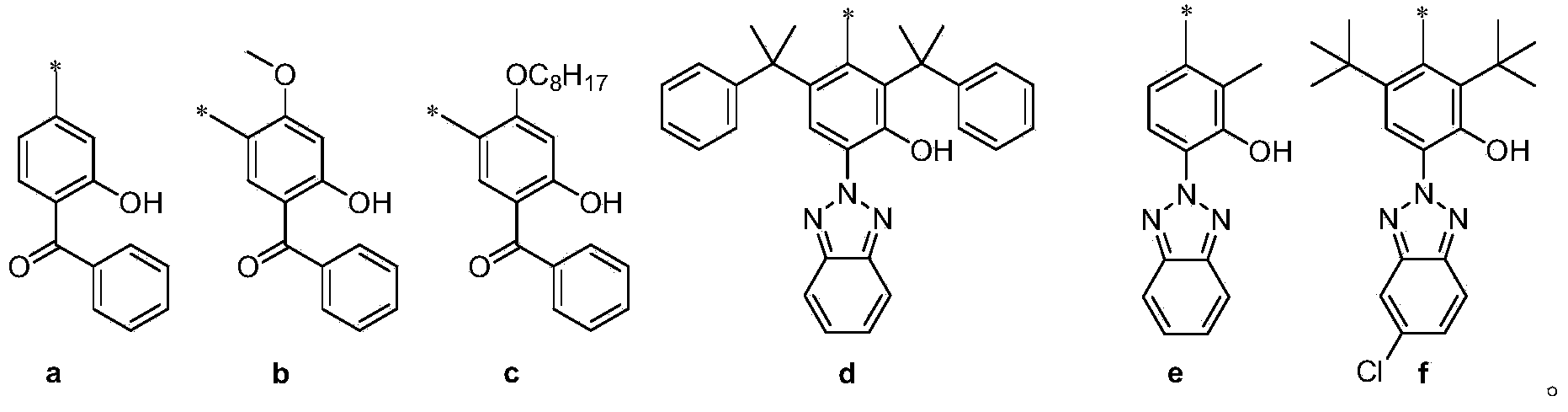
[0140] * Note: product number 3a refers to the compound of formula 3 where R is corresponding to formula a, and so on.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com