Silane terminated polyurethane oligomer and its preparation method
A polyurethane oligomer and silane-terminated technology, which is applied in the field of modified polyurethane, can solve the problems of difficult synthesis process, difficult thick coating process, easy occurrence of gel and the like, and achieves convenient construction, simple and easy preparation conditions, and easy availability of raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
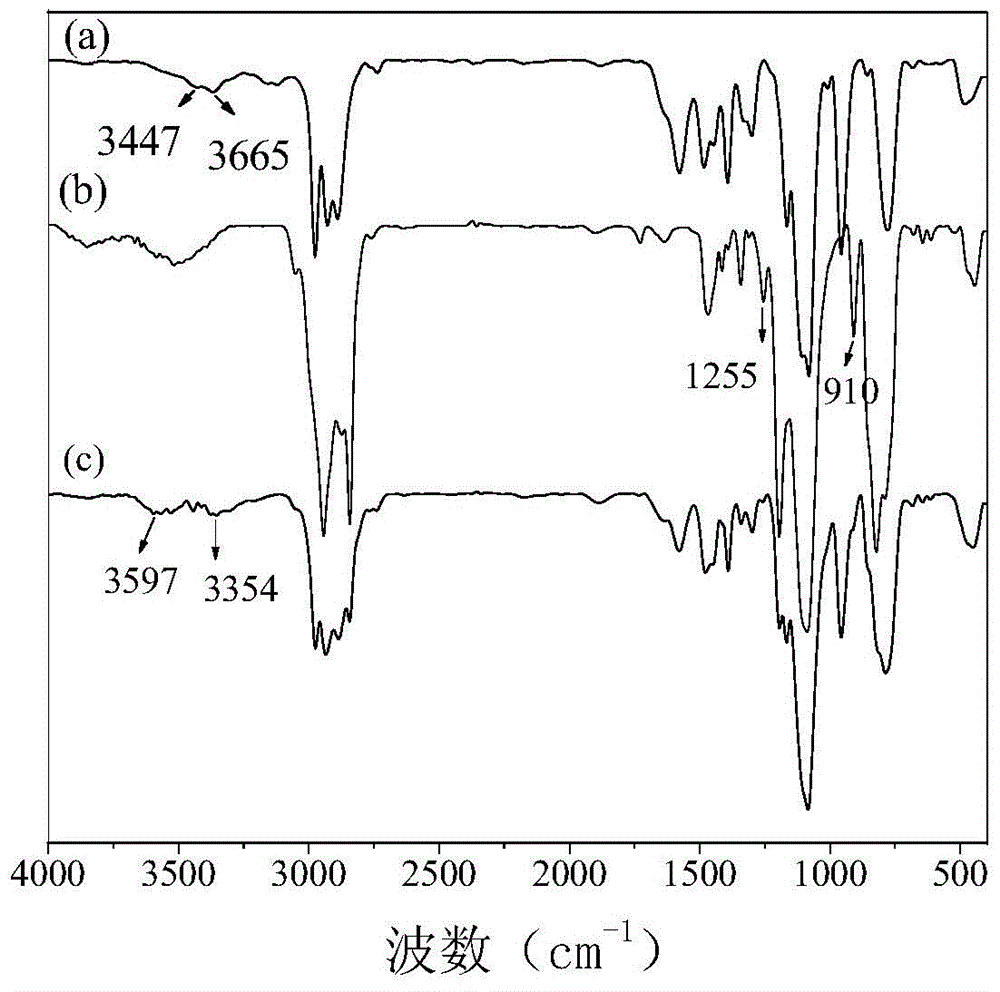
[0044] (1) Preparation of secondary aminosilane A: In terms of parts by mass, put 22.14 parts of γ-aminopropyltriethoxysilane in the reaction flask, and then according to the molar ratio of 1:1, slowly Add 22.03 parts of γ-(2,3-glycidoxy)propylmethyldimethoxysilane dropwise, and then control the system to stir and react at 20°C for 12 hours to obtain secondary aminosilane A;
[0045] (2) Dehydrate and degas 17 parts of polyether polyol (N220, Foshan Shunde Xingzhou Synthetic Materials Co., Ltd.) at 110°C under vacuum for 4 hours, then cool down to 70°C, and then add 3 parts of 1,6-hexyl Diol, 12 parts of xylene, 9 parts of TDI, and 0.2 parts of dibutyltin dilaurate were added to polyether polyol N220, and high-purity nitrogen was introduced as a reaction protection gas, stirred evenly, and then kept at 80°C for 4 hours. One-step synthesis of isocyanate-terminated polyurethane prepolymers;
[0046] (3) React the isocyanate-terminated polyurethane prepolymer synthesized in step...
Embodiment 2
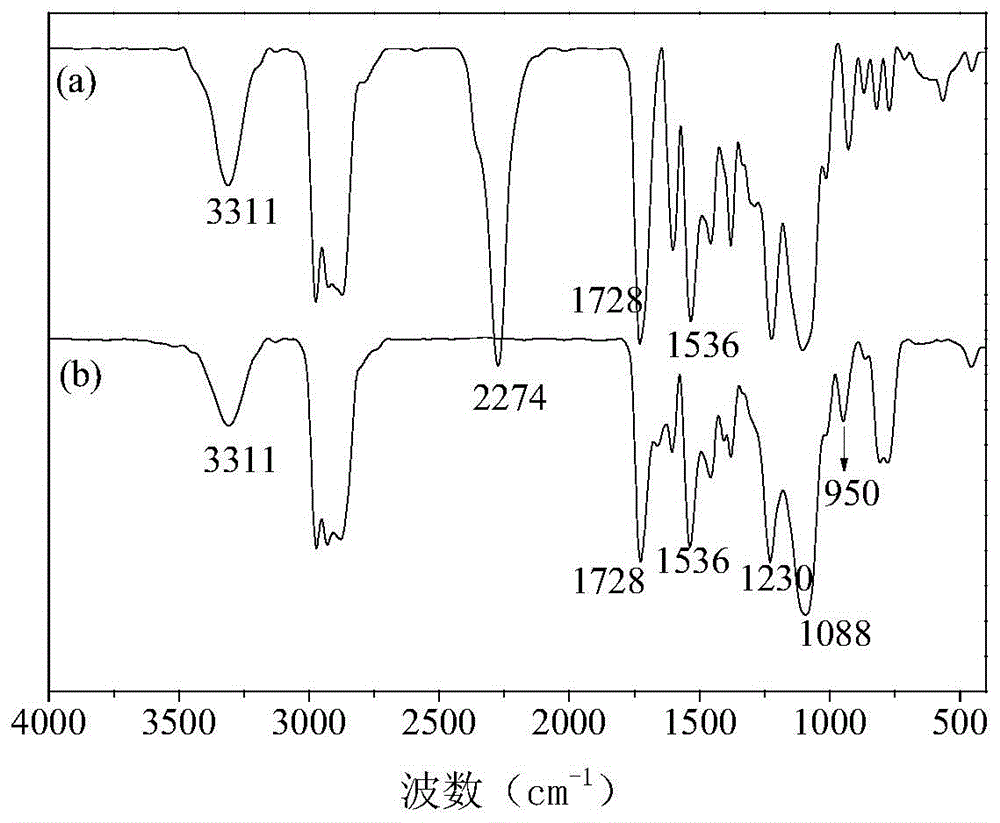
[0050] (1) Preparation of secondary aminosilane B: In terms of mass fraction, put 22.14 parts of γ-aminopropylmethyldiethoxysilane in the reaction flask, and then according to the molar ratio of 1:1, under the condition of stirring Slowly add 29.04 parts of γ-methacryloxypropyltriethoxysilane dropwise, and then control the system to stir and react at 60°C for 168 hours to obtain secondary aminosilane B;
[0051] (2) Dehydrate and degas 17 parts of polyether polyol (N220, Foshan Shunde Xingzhou Synthetic Materials Co., Ltd.) at 110°C for 3 hours, then cool down to 70°C, and then add 2.25 parts of 1,4-butyl Diol, 12 parts of butyl acetate, 9 parts of TDI, and 0.4 parts of dibutyltin dilaurate were added to polyether polyol N220, and high-purity nitrogen gas was introduced as a reaction protection gas, stirred evenly, and then kept at 85°C for 3.5 h, Synthesis of isocyanate-terminated polyurethane prepolymer by one-step method;
[0052] (3) react the isocyanate-terminated polyur...
Embodiment 3
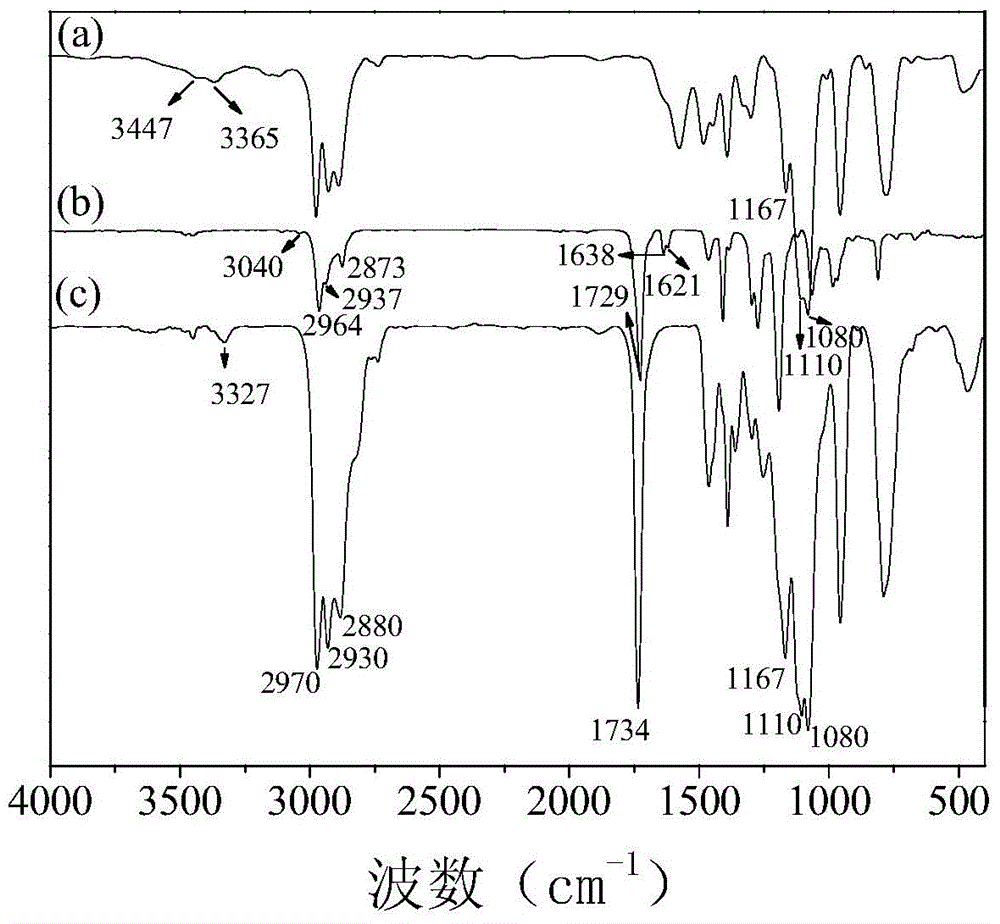
[0055] (1) Preparation of secondary aminosilane C: in terms of mass fraction, put 17.93 parts of γ-aminopropyltrimethoxysilane in the reaction flask, and slowly dropwise under the condition of stirring according to the molar ratio of 1:1 Add 24.83 parts of γ-methacryloxypropyltrimethoxysilane, and then control the system to stir and react at 50°C for 120h to obtain secondary aminosilane C;
[0056] (2) Dehydrate and degas 17 parts of polyether polyol (N220, Foshan Shunde Xingzhou Synthetic Materials Co., Ltd.) at 120°C for 3 hours, then cool down to 60°C, and then add 2.25 parts of 1,4-butyl Diol, 12 parts of ethyl acetate, 13 parts of MDI, and 0.6 parts of dibutyltin dilaurate were added to polyether polyol N220, and high-purity nitrogen was introduced as a reaction protection gas, stirred evenly, and then kept at 85°C for 3 hours. , by one-step synthesis of isocyanate-terminated polyurethane prepolymer;
[0057] (3) React the isocyanate-terminated polyurethane prepolymer sy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com