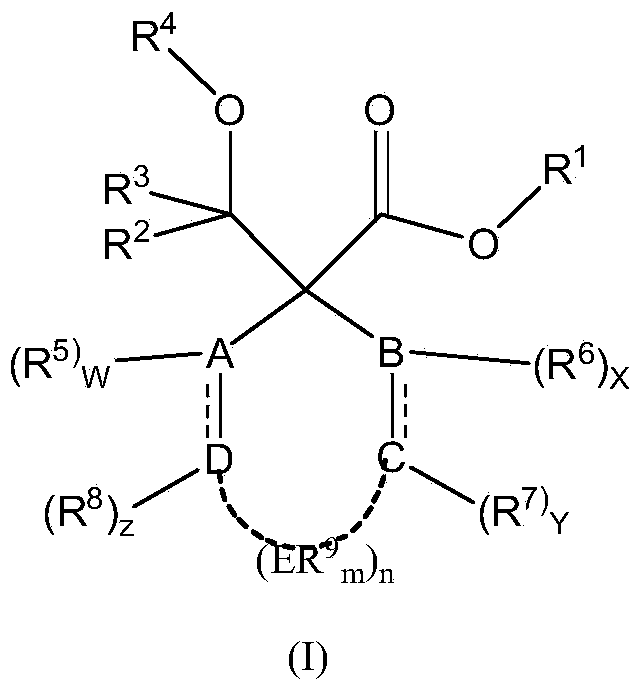
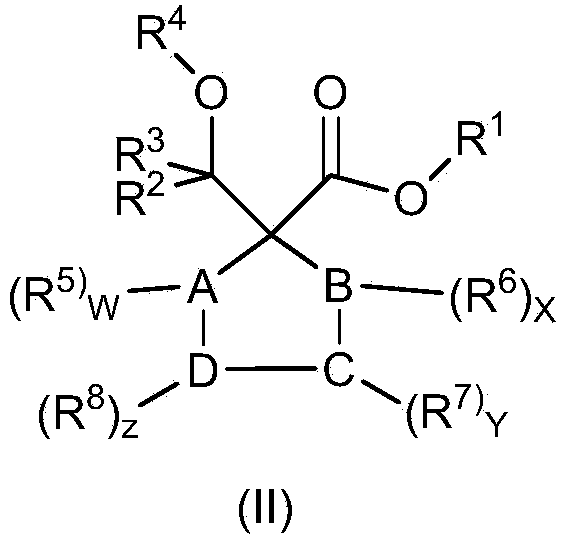
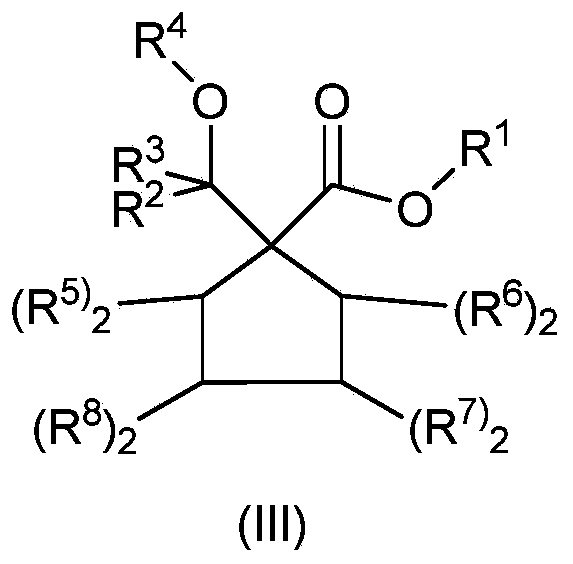
Catalyst component for olefin polymerization and catalyst
A technology of olefin polymerization and solid catalyst, applied in the field of monofunctional or multifunctional ketones or amines, which can solve the unsatisfactory balance of activity/isotacticity and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 19
[0131] Example 19 Synthesis of Methoxymethyl-Fluorenic Acid-(9)-Methyl Ester
[0132] Step A: Under the protection of nitrogen, add 18g sodium hydride, 50g fluorene, and 150mL toluene into a 1000mL three-necked flask, turn on mechanical stirring, raise the temperature to 125°C and reflux, keep the reaction for 4h; lower the temperature to 90°C, and slowly drop 146.1 into the flask g diethyl carbonate, drip in 1.5h, continue to react for 3h after dripping; cool to 20℃, slowly drip in a mixture of 60g concentrated hydrochloric acid and 75g water, and control the temperature not to exceed 40℃; filter to separate organic Wash the phase with water until it is neutral. The organic phase will be vortexed to obtain a reddish-brown liquid; the fluid obtained by the rotatory steaming will be refluxed with 157.4g of acetic acid and 63g of 10% hydrochloric acid overnight; the mixture will be reduced to 20℃, and the liquid will be separated; Then, 30% NaOH solution was added to adjust the pH ...
Embodiment 29
[0136] Example 29 Synthesis of methoxymethyl-fluorenic acid-(9)-ethyl ester
[0137] The synthesis steps are the same as in Example 1, except that the methanol in step B is replaced with ethanol. 1 H-NMR(CDCl 3 )δ (ppm): 1.17-1.20 (t, 3H, methyl hydrogen), 3.37 (s, 3H, ether methyl hydrogen), 3.791 (s, 2H, ether methylene hydrogen), 4.14 to 4.19 (m, 2H, ester methylene hydrogen), 7.26-7.42 (t, 2H, aromatic ring hydrogen), 7.42-7.44 (t, 2H, aromatic ring hydrogen), 7.73-7.74 (m, 4H, aromatic ring hydrogen).
Embodiment 39
[0138] Example 39 Synthesis of Ethoxymethyl-fluorenic acid-(9)-ethyl ester
[0139] The synthesis procedure is the same as in Example 1, except that the methanol in step B is replaced with ethanol, and the chloromethyl methyl ether in step C is replaced with chloromethyl ethyl ether. 1 H-NMR(CDCl 3 )δ(ppm): 1.13-1.17 (t, 3H, ether methyl hydrogen), 1.30-1.34 (t, 3H, ester methyl hydrogen), 3.40-3.46 (m, 2H, ether methylene hydrogen), 3.90 (s, 2H, ether methylene hydrogen), 4.12-4.16 (m, 2H, ester methylene hydrogen), 7.26-7.40 (t, 2H, aromatic ring hydrogen), 7.41-7.43 (t, 2H, aromatic ring Hydrogen), 7.72-7.74 (m, 4H, aromatic ring hydrogen).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com