Preparation method of cadmium arsenide semiconductor nanocluster
A technology of nanoclusters and semiconductors, which is applied in the field of synthesis of a large number of cadmium arsenide nanoclusters, which can solve the problems of few cadmium arsenide nanoclusters, difficult storage, cumbersome methods, etc., and achieve good monodispersity, good luminescent properties, and reaction Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] First, prepare the cadmium oleate solution. Take 12mmol (1.536g) of cadmium oxide powder, 25mmol (8.75mL) of OA (oleic acid) and 11.25mL of ODE (octadecene) and mix, heat to 250 ℃ under nitrogen protection to dissolve cadmium oxide, and cool to 120 ℃, prepared into cadmium oleate solution, a light yellow transparent solution.
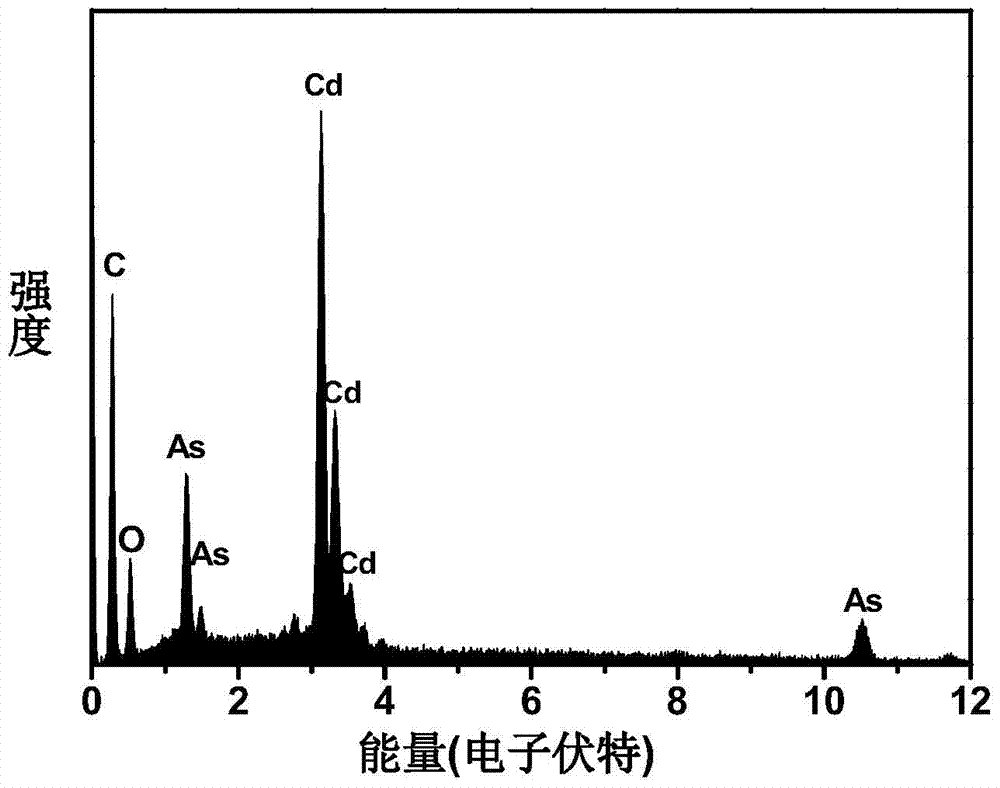
[0023] Then take 1mmol (0.2228g) of magnesium arsenide into the reaction flask, and inject 1.5mL of hydrochloric acid with a concentration of 4mol / L. The gas produced by the reaction is passed into the above 120℃ cadmium oleate solution and reacted for 30 minutes to obtain Cd 3 As 2 Cluster compound.
Embodiment 2
[0025] First, prepare the cadmium myristate solution. Take 12mmol (1.536g) of cadmium oxide powder, 25mmol of myristic acid and 11.25mL of ODE (octadecene) and mix, heat to 250°C under nitrogen protection to dissolve cadmium oxide, and cool to 50°C to prepare myristic acid Cadmium solution is a colorless and transparent solution.
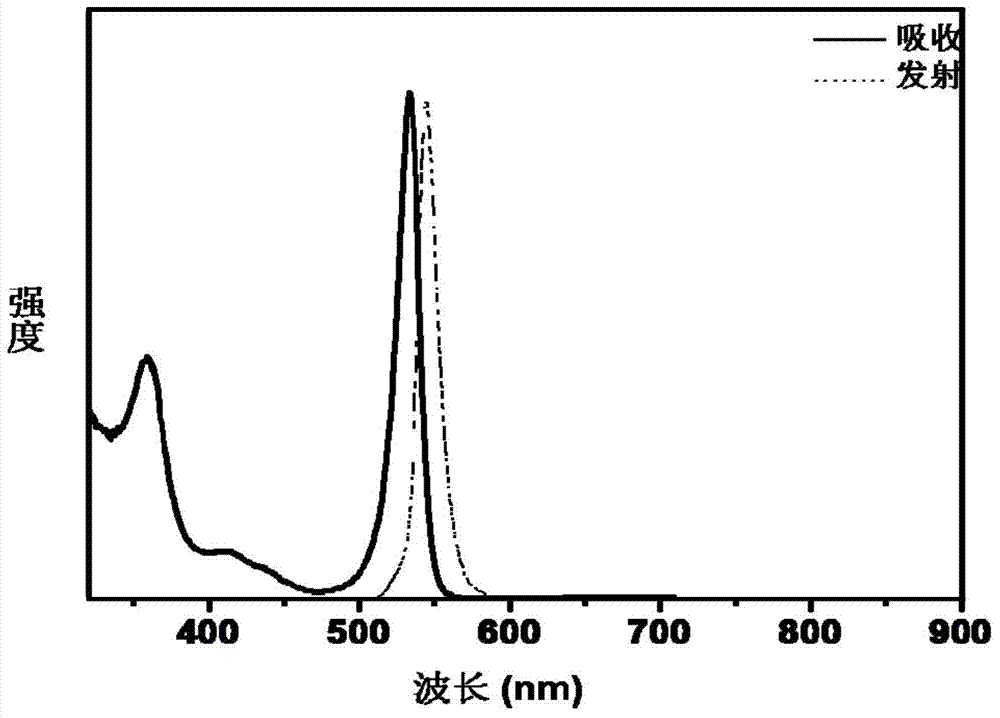
[0026] Then take 1mmol zinc arsenide into the reaction flask, and inject 1.5mL sulfuric acid with a concentration of 4mol / L. The gas produced by the reaction is passed into the above-mentioned 50℃ cadmium myristate solution and reacted for 30 minutes to obtain the Cd 3 As 2 The ultraviolet-visible absorption peaks of the cluster compounds are sharp and symmetrical, the fluorescence peaks are sharply symmetrical, and the half-peak width is very narrow, showing a good size distribution.
Embodiment 3
[0028] First, prepare the cadmium oleate solution. Take 12mmol (1.536g) of cadmium oxide powder, 25mmol of OA (oleic acid, 8.75ml) and 91.25mL of ODE (octadecene) and mix, heat to 250℃ under nitrogen protection to dissolve the cadmium oxide, and cool to 120℃ , Preparation of cadmium oleate solution, a light yellow transparent solution.
[0029] Take 2mmol of magnesium arsenide into the reaction flask, and inject 10mL of hydrochloric acid with a concentration of 4mol / L. The gas produced by the reaction is passed into the above-mentioned 120℃ cadmium oleate solution and reacted for 30 minutes to obtain Cd 3 As 2 Compared with the above-mentioned examples, the cluster compound exhibits a slightly higher concentration and more products are obtained, and the spectral effect is still symmetrical and sharp.
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com