Preparation method of boron-11 acid with high abundance
A high-abundance, boron trifluoride technology, applied in the field of preparation of high-abundance boron-11 acid, can solve the problems of increasingly high performance requirements of semiconductor devices, negative impact on the performance of semiconductor devices, crashes, etc. Excellent anti-interference and anti-radiation performance, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
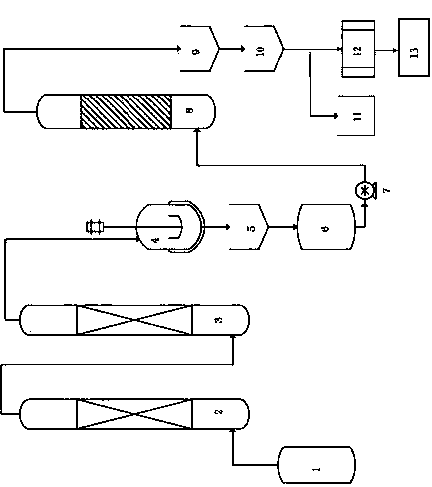
[0038] A method for preparing high-abundance boron-11 acid, comprising the following steps:
[0039] (i) Purification of high-abundance boron trifluoride-11 as raw material
[0040] The high-abundance boron trifluoride-11 in the raw material storage tank 1 is passed through the first purification tower 2 to remove entrained complexes and the second purification tower 3 to remove light components of the air under the conditions of 25°C and 1.0 MPa , to obtain pure boron trifluoride-11 gas;
[0041] (ii) Hydrolysis of boron trifluoride-11
[0042] Pass the purified boron trifluoride-11 gas into the lithium hydroxide aqueous solution heated to 50-90°C in the hydrolysis kettle 4, and the pure boron trifluoride-11 will be hydrolyzed with water to generate boron-11 acid and hydrogen fluoride, Fluoride ions are further combined with lithium ions in the solution to form lithium fluoride precipitates,
[0043] The reaction formula is as follows:
[0044] 11 BF 3 +3H 2 O→H 3 11...
Embodiment 1
[0062](i) Purification of high-abundance boron trifluoride-11 as raw material
[0063] The high-abundance boron trifluoride-11 in the raw material storage tank 1 is passed through the first purification tower 2 to remove entrained complexes and the second purification tower 3 to remove light components of the air under the conditions of 25°C and 1.0 MPa , to obtain pure boron trifluoride-11 gas;
[0064] (ii) Hydrolysis of boron trifluoride-11
[0065] Pass the purified boron trifluoride-11 gas into the lithium hydroxide aqueous solution heated to 50°C in the hydrolysis kettle 4, wherein the theoretically calculated amount of lithium hydroxide is 1%, and pure boron trifluoride-11 will generate The hydrolysis reaction produces boron-11 acid and hydrogen fluoride, and the fluoride ions are further combined with lithium ions in the solution to form lithium fluoride precipitates.
[0066] When no more precipitation is generated, stop feeding the high-abundance boron trifluoride-...
Embodiment 2
[0079] (i) Purification of high-abundance boron trifluoride-11 as raw material
[0080] The high-abundance boron trifluoride-11 in the raw material storage tank 1 is passed through the first purification tower 2 to remove entrained complexes and the second purification tower 3 to remove light components of the air under the conditions of 25°C and 1.0 MPa , to obtain pure boron trifluoride-11 gas;
[0081] (ii) Hydrolysis of boron trifluoride-11
[0082] Pass the purified boron trifluoride-11 gas into the lithium hydroxide aqueous solution heated to 80°C in the hydrolysis kettle 4, wherein the theoretically calculated amount of lithium hydroxide is 5%, and pure boron trifluoride-11 will generate The hydrolysis reaction produces boron-11 acid and hydrogen fluoride, and the fluoride ions are further combined with lithium ions in the solution to form lithium fluoride precipitates.
[0083] When no more precipitation is generated, stop feeding the high-abundance boron trifluoride...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com