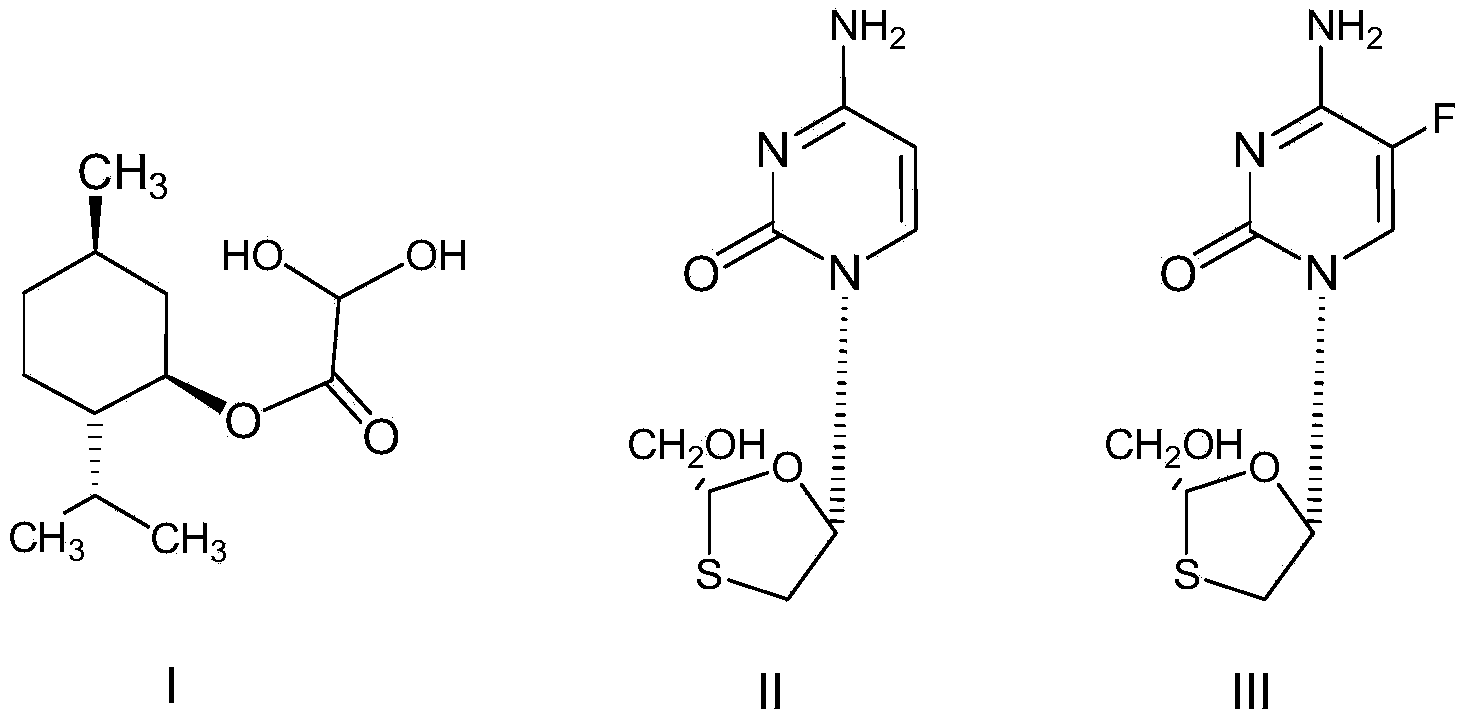
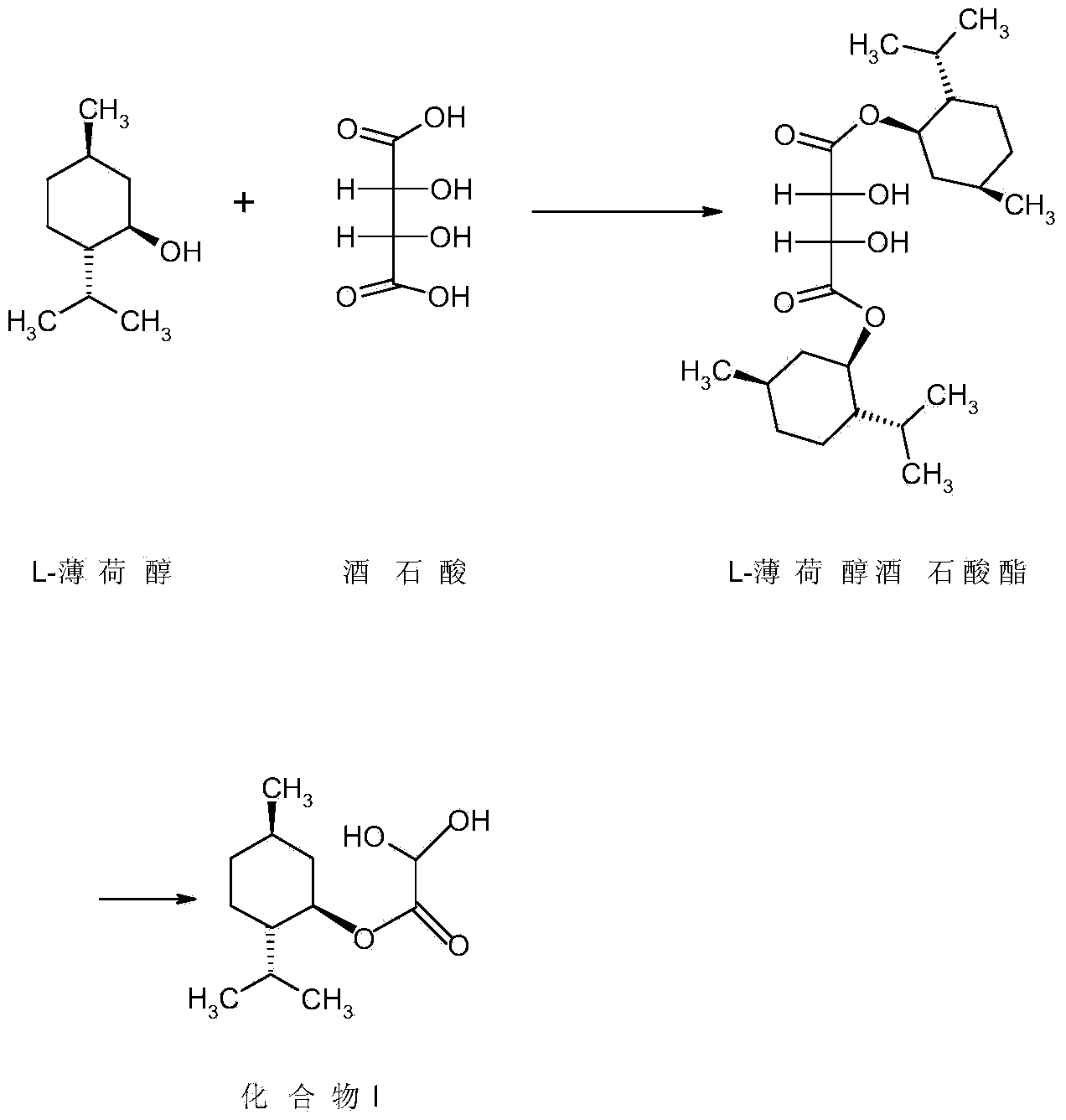
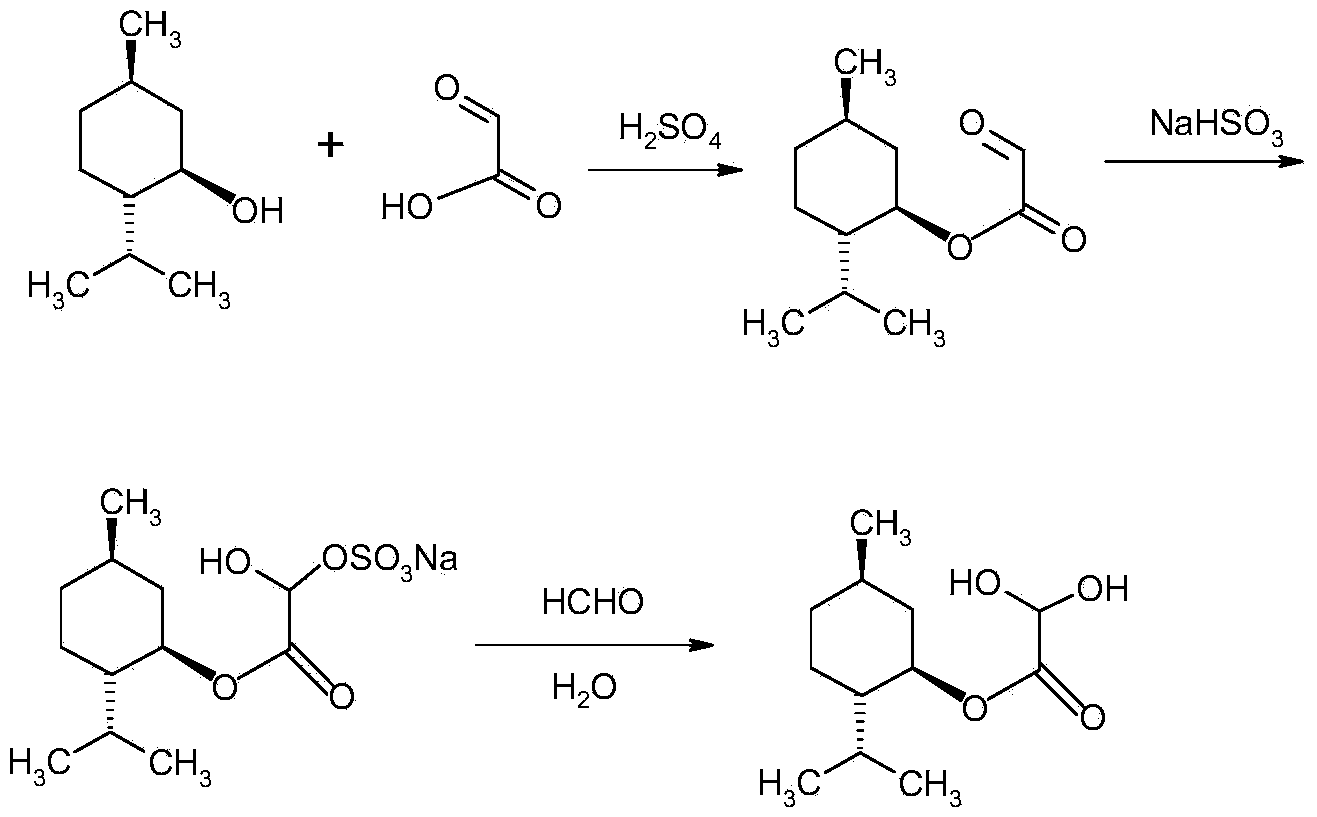
Method for preparing L-menthyl glyoxylate-hydrate through catalysis of heteropoly acid
A technology of menthol glyoxylate and monohydrate, applied in the field of medicine, can solve problems such as poor solubility of solid acid, many by-products, complicated reaction, etc., and achieves reduction of discharge of waste acid and salt, improvement of catalytic activity, and improvement of products. good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Catalytic preparation of L-menthol glyoxylate monohydrate by phosphotungstic acid
[0036] Step 1: Add 50 grams of cyclohexane and 57 grams (0.365 mol) of L-menthol into a 250 mL three-necked flask equipped with a stirrer and a thermometer, and stir at about 25°C until completely dissolved. After the addition, install the water separator and condenser, stir and heat the reactant slowly, add 0.5 g of phosphotungstic acid, slowly add 17.6 g of glyoxylic acid with a mass fraction of 50% dropwise under reflux and stirring The aqueous solution (containing 8.8 g of glyoxylic acid) was refluxed for 5 hours. The water taken out is separated by the water separator.
[0037]Step 2: After the reaction is completed, cool down, filter to remove phosphotungstic acid, add sodium sulfite aqueous solution (5 g sodium sulfite (0.040 mol) + 45 g water) to the separated organic layer, and add sodium bisulfite dropwise within 20 to 30 minutes The aqueous solution (8.2 g sodium ...
Embodiment 2
[0039] Example 2: Catalytic preparation of L-menthol glyoxylate monohydrate by phosphotungstic acid
[0040] Use the phosphotungstic acid obtained after the first step of liquid separation in Example 1 to continue to use, no need to add phosphotungstic acid, and operate according to Example 1 to obtain 20.4 grams of product, yield: 72.8%, GC content: 99.1%.
Embodiment 3
[0041] Example 3: Preparation of L-menthol glyoxylate monohydrate catalyzed by silicotungstic acid
[0042] Use 0.5 g of silicotungstic acid instead of phosphotungstic acid, and operate according to Example 1 to obtain a product: 23.4 g, yield: 83.5%, GC content: 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com