Synthesis method of 1,2-benzoisothiazolinyl-3-one compound
A technology of benzisothiazoline and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of long process steps, poor product quality, serious pollution, etc., and achieve the effect of simple post-processing steps, less environmental pollution, and simple process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
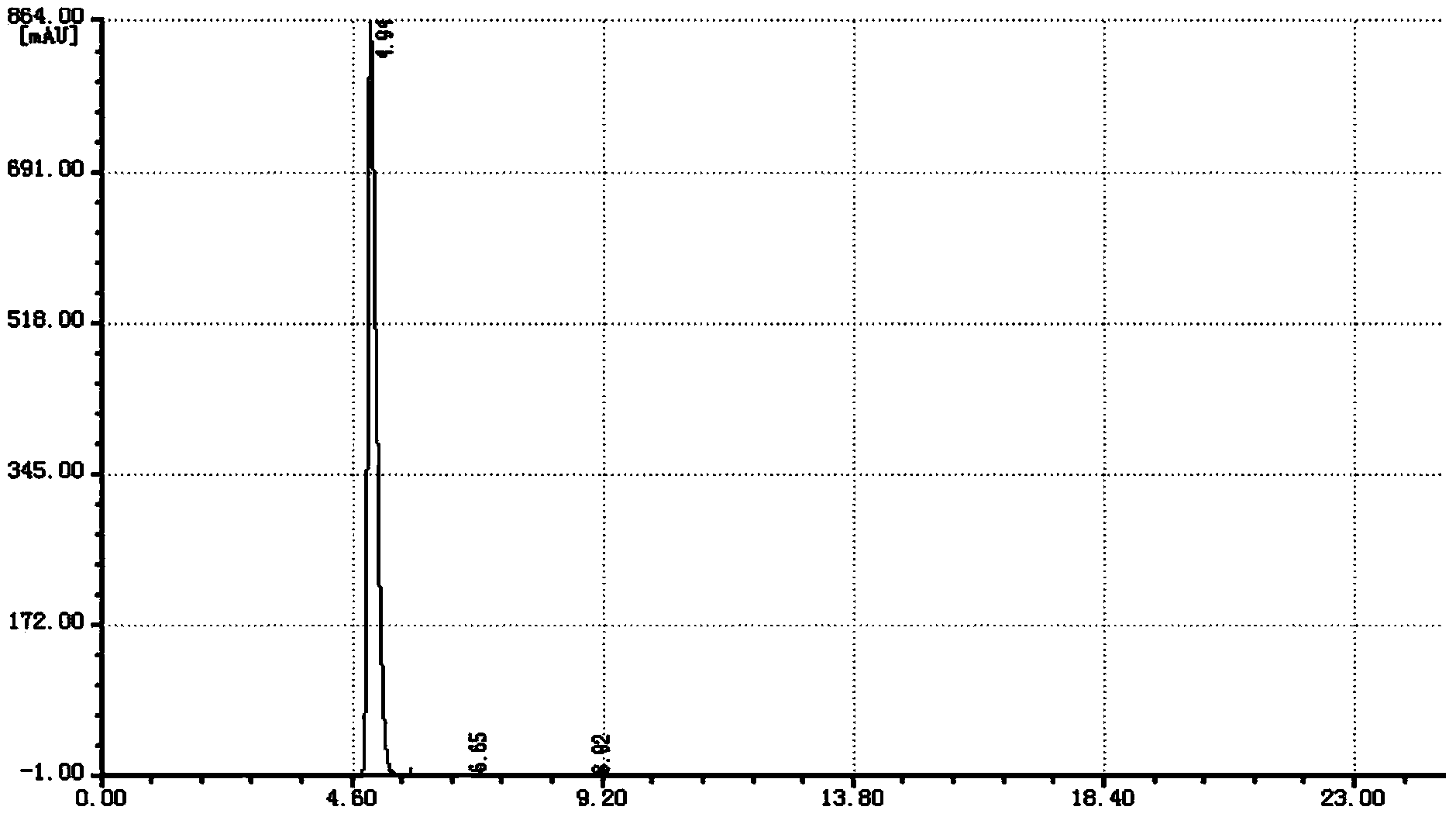
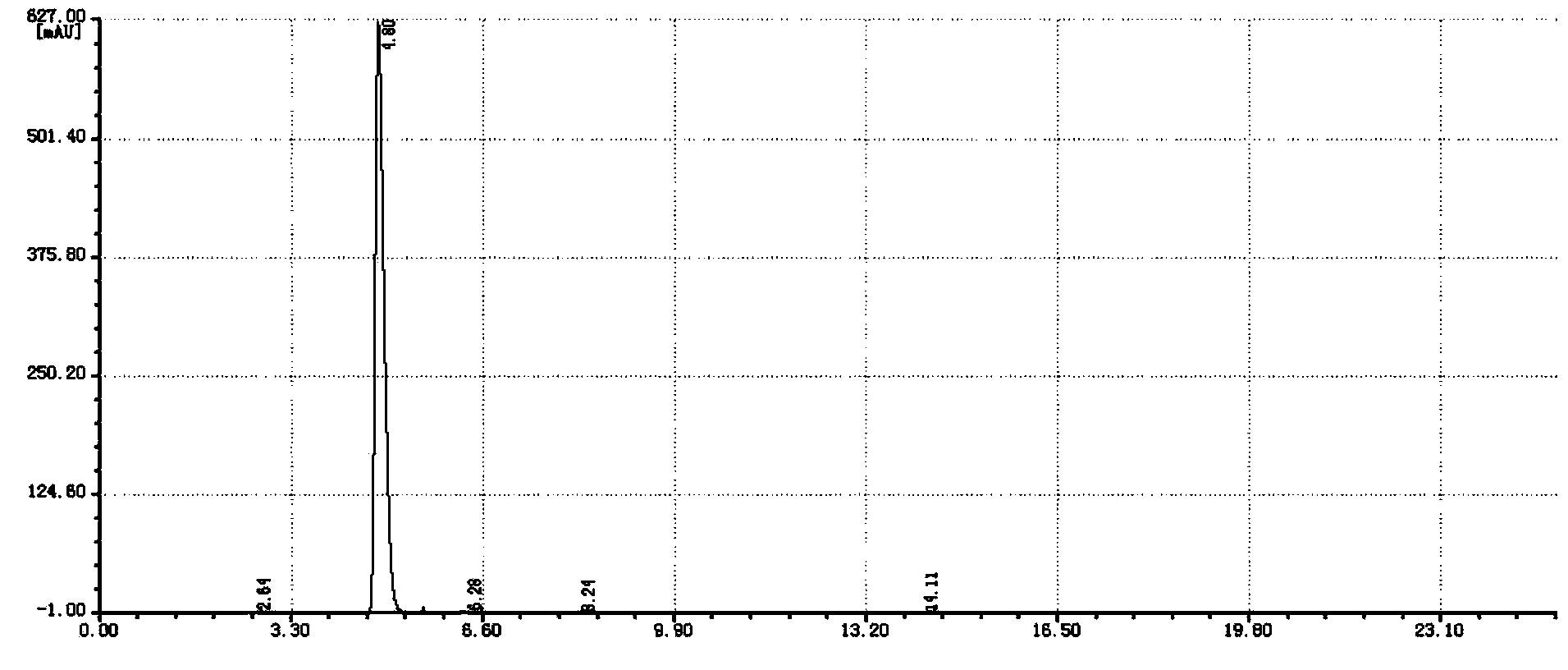
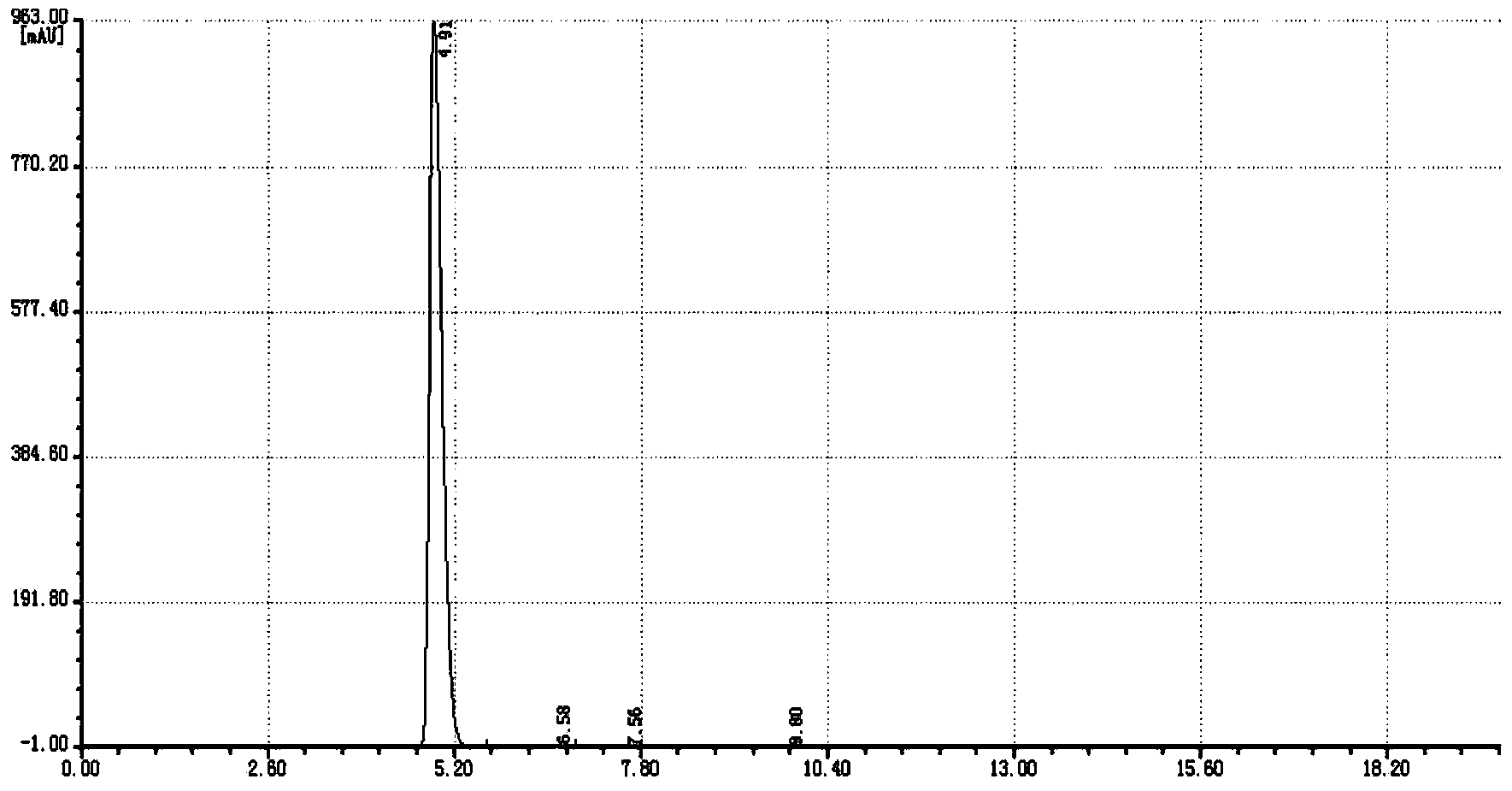
Image
Examples
Embodiment 1
[0030] Under a nitrogen atmosphere, 100 g of chlorobenzene, 100 g of o-chlorobenzonitrile, 219 g of octadecylmercaptan, and 4 g of tetrabutylammonium bromide were added to a 1000 ml four-necked flask equipped with a stirrer, a thermometer and a condenser. At 65-70°C, 98.7g of 32% sodium hydroxide solution was added dropwise, and the reaction was incubated until it was qualified, and the water phase was separated to obtain 381g of o-octadecylthiobenzonitrile reaction solution.
[0031] In a 1000ml four-necked flask equipped with a stirrer, a thermometer and a condenser, 381g of o-octathiocyanil benzonitrile reaction solution, 100g of chlorobenzene and 32g of water were added. At 20-30°C, 53g of chlorine gas was introduced. Raise the temperature to 60-65°C and react for 1h. Cool down to 20-30°C, add 200g of water, adjust the pH to 9-10 with 32% liquid caustic soda, raise the temperature to 60-65°C, remove the organic phase, cool the water phase to 20-30°C, add 31% hydrochloric ...
Embodiment 2
[0033] Under a nitrogen atmosphere, 100 g of chlorobenzene, 100 g of o-chlorobenzonitrile, 112 g of n-octyl mercaptan, and 4 g of tetrabutylammonium bromide were added to a 500 ml four-necked flask equipped with a stirrer, a thermometer and a condenser. At 60-65° C., 98 g of 32% sodium hydroxide solution was added dropwise, and the reaction was kept until it was qualified, and the water phase was separated to obtain 279 g of o-octylthiobenzonitrile reaction liquid.
[0034] In a 1000ml four-neck flask equipped with a stirrer, a thermometer and a condenser, 279g of o-octionitrile benzonitrile reaction solution, 200g of chlorobenzene and 30g of water were added. At 20-30°C, 54g of chlorine gas was introduced. Raise the temperature to 60-65°C and react for 1h. Cool down to 20-30°C, add 200g of water, adjust the pH to 9-10 with 32% liquid caustic soda, raise the temperature to 60-65°C, separate the organic phase, separate the organic phase to recover the solvent and by-products, ...
Embodiment 3
[0036] Under a nitrogen atmosphere, 100 g of chlorobenzene, 100 g of o-chlorobenzonitrile, 154 g of n-dodecanethiol, and 4 g of tetrabutylammonium bromide were added to a 500 ml four-necked flask equipped with a stirrer, a thermometer and a condenser. At 60-65°C, 98g of 32% sodium hydroxide solution was added dropwise, and the reaction was kept until it was qualified, and the water phase was separated to obtain 320g of o-dodecylthiobenzonitrile reaction solution.
[0037]In a 1000ml four-necked flask equipped with a stirrer, a thermometer and a condenser, 320 g of o-dodecylthionitrile benzonitrile reaction solution, 200 g of chlorobenzene, and 30 g of water were added. At 20-30°C, 54g of chlorine gas was introduced. Raise the temperature to 60-65°C and react for 1h. Cool down to 20-30°C, add 200g of water, adjust the pH to 9-10 with 32% liquid caustic soda, raise the temperature to 60-65°C, separate the organic phase, separate the organic phase to recover the solvent and by-p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com