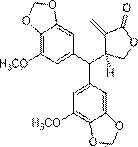
Semisynthesis method of antineoplastic activity natural product peperomin E
A technology for antitumor activity and oxaliphacin is applied in the field of semi-synthesis of antitumor active natural product oxalicum E, which can solve the problems of high cost, low content, poor product purity and the like, achieve mild conditions, expand sources and simple methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
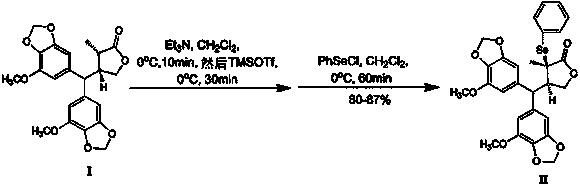
[0020] (3 R , 4 R )-2-Phenylselenyl oxalonine A (II) preparation
[0021] Dissolve boronine A (I) (5g, 12mmol) in triethylamine (22.5mL, 162mmol), stir at 0°C for 10min, add trimethylsilyl trifluoromethanesulfonate (25mL, 138mmol), continue After stirring at 0°C for 40min, add a chloroform solution (50mL) containing phenylselenyl chloride (9.5g, 50.0mmol) and stir at 0°C for 30min. 2 Carried out under gas protection. After adding 500mL of saturated ammonium chloride solution to the reaction solution, extract it with ethyl acetate (800mL) for 3 times, combine the extracts, recover ethyl acetate, perform silica gel column chromatography [200-300 mesh silica gel, petroleum ether-ethyl acetate ( Volume ratio 4:1)] refined to get (3 R , 4 R )-2-Phenylselenoylporonin A(II) (5.95g, 87% yield): m.p. 77-79 o C; [α] 20 D +50.1 (c 0.40, CHCl 3 ); ESI-MS m / z 593.0676 [M+Na] + , 1 H-NMR δ H 7.60(2H, m, m -PhSe), 7.39(1H, m, p -PhSe), 7.29(2H, m, o -PhSe), 6.45(1H, br...
Embodiment 2
[0025] (3 R , 4 R )-2-Phenylselenyl oxalonine A (II) preparation
[0026] Dissolve boronine A (I) (15g, 36mmol) in triethylamine (70mL, 0.50mol), stir at 0°C for 10min, then add trimethylsilyl trifluoromethanesulfonate (80mL, 0.44mol), After continuing to stir at 0°C for 40 min, add a chloroform solution (150 mL) containing phenylselenyl chloride (30 g, 0.16 mol) and stir at 0°C for 30 min. 2 Carried out under gas protection. After adding 1000mL saturated ammonium chloride solution to the reaction solution, extract it with ethyl acetate (1.5L) for 3 times, combine the extracts, recover ethyl acetate, perform silica gel column chromatography [200-300 mesh silica gel, petroleum ether-ethyl acetate (volume ratio 4:1)] refined to get (3 R , 4 R )-2-Phenylselenoylporonin A(II) (16.9 g, yield 82.4%).
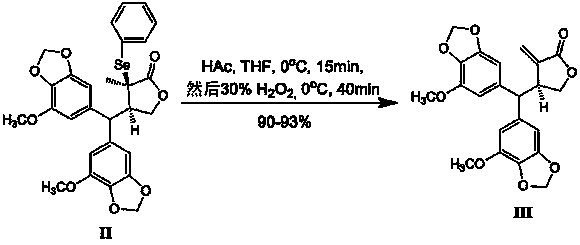
[0027] Preparation of Herbaperine E(III)
[0028] Will (3 R , 4 R )-2-Phenylselenoylpiperonin A(II) (10g, 18mmol) was dissolved in tetrahydrofuran (180mL), added glacial acet...
Embodiment 3
[0030] (3 R , 4 R )-2-Phenylselenyl oxalonine A (II) preparation
[0031] Dissolve boronine A (I) (200g, 0.48mol) in triethylamine (1000mL, 7.2mol), stir at 0°C for 20min, then add trimethylsilyl trifluoromethanesulfonate (1000mL, 5.5mol) , after continuing to stir at 0°C for 40min, add a chloroform solution (1000mL) containing phenylselenyl chloride (400g, 2.08mol), and stir at 0°C for 40min. 2 Carried out under gas protection. After adding 8 L of saturated ammonium chloride solution to the reaction solution, extract it three times with ethyl acetate (10 L), combine the extracts, recover ethyl acetate, perform silica gel column chromatography [200-300 mesh silica gel, petroleum ether-ethyl acetate (volume ratio 4:1)] refined to get (3 R , 4 R )-2-Phenylselenoylporonin A(II) (219.2 g, yield 80.1%).
[0032] Preparation of Herbaperine E(III)
[0033] Will (3 R , 4 R )-2-Phenylselenoylpiperonin A(II) (100 g, 0.18mol) was dissolved in tetrahydrofuran (1000m L), added gla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com