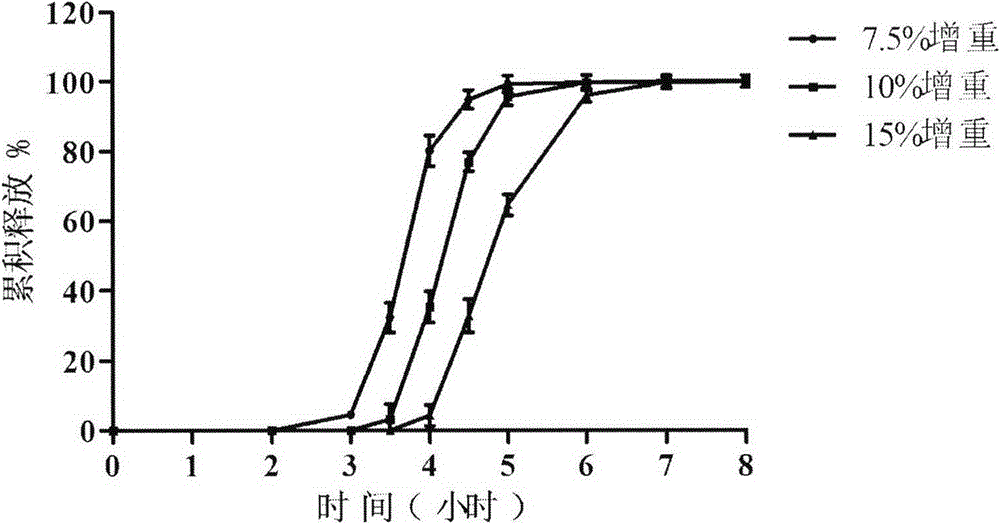
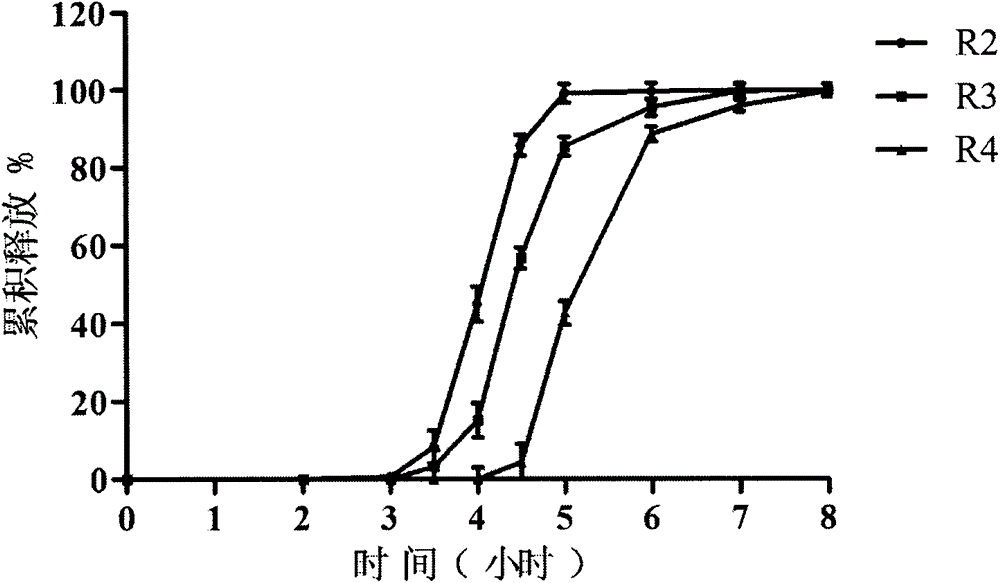
Zolpidem tartrate oral pulse controlled-release delivery system and preparation method thereof
A zolpidem tartrate and drug delivery system technology, which is applied in the fields of pharmaceutical formulations, nervous system diseases, drug combinations, etc., can solve the difficulties of process control, large batch-to-batch variation, and no literature and patent reports on oral pulse-controlled release preparations, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] R 1 Tablet prescription:
[0042]
[0043] Preparation Process:
[0044] (1) Preparation of Submicron Solid Dispersion Drugs
[0045] A. Preparation of micronized medicine: take 55g zolpidem tartrate raw material, put in jet mill, set powder delivery pressure as 4bar, grinding pressure 2bar, powder delivery speed 0.5g / min, pulverization time is 30min, particle is carried out Micronized pulverization, continuous pulverization twice to obtain micronized drug (average particle size 0.5-3 μm), the yield is 91%;
[0046] B. the preparation of submicron solid dispersion medicine: take by weighing 50g micronized medicine, and 290g solid dispersion carrier lactose ( 200) Place a cube mixer at a rotational speed of 180rpm, and mix for 10 minutes; take the solid dispersion mixed drug and put it in a small ball mill, add zirconia balls, and grind for 2 hours to obtain a submicron solid dispersed drug. Its scanning electron microscope picture is shown in figure 1 .
[004...
Embodiment 2
[0055] R 2 Tablet prescription:
[0056]
[0057] Preparation Process:
[0058] (1) Preparation of Submicron Solid Dispersion Drugs
[0059] A. Preparation of micronized medicine: take 53g zolpidem tartrate raw material, put in the jet mill, set the powder conveying pressure as 5bar, grinding pressure 3bar, powder conveying speed 0.6g / min, crushing time is 35min, the particle is carried out Micronized pulverization, continuous pulverization twice to obtain micronized drug (average particle size 0.2-5 μm), the yield is 94%;
[0060] B. Preparation of submicron solid dispersion drug: Weigh 50g of micronized drug, put it in a cube mixer with 250g of solid dispersion carrier compressible starch, and mix it for 15min at a speed of 150rpm; take the solid dispersion mixed drug and put it in a small ball mill, add Zirconia grinding balls, grind for 3 hours to obtain submicron solid-dispersed drugs.
[0061] (2) Preparation of drug-containing tablet core in solubilization system...
Embodiment 3
[0069] R 3 Tablet prescription:
[0070]
[0071] Preparation Process:
[0072] (1) Preparation of Submicron Solid Dispersion Drugs
[0073] Preparation of micronized drug: Weigh 54g zolpidem tartrate raw material, put it in the jet mill, set the powder delivery pressure to 6bar, the grinding pressure to 3bar, the powder delivery speed to 0.3g / min, and the pulverization time to 35min, and the particles are micronized Pulverize, pulverize twice in a row to obtain micronized drug (average particle size 0.5-8 μm), yield is 92.5%;
[0074] Preparation of submicron solid-dispersed drug: Weigh an appropriate amount of micronized drug, put it in a cube mixer with solid dispersion carrier lactose (Tablettose80), and mix it for 20 minutes at a speed of 150 rpm; take the solid-dispersed mixed drug and put it in a small ball mill, add zirconia mill balls, and grind for 4 hours to obtain submicron solid-dispersed drugs.
[0075] (2) Preparation of solubilizing system drug-containin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com