Zolpidem tartrate oral pulse controlled release drug delivery system and preparation method thereof
A zolpidem tartrate, drug delivery system technology, applied in the pulse controlled release composition and its tablet, the field of time-selected controlled release preparations for the treatment of early awakening and sleep disorders, can solve the problem of large batch-to-batch differences, no oral pulse controlled release preparation literature and patent reports, difficulty in process control, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] R 1 Tablet prescription:
[0042]
[0043] Preparation Process:
[0044] (1) Preparation of Submicron Solid Dispersion Drugs
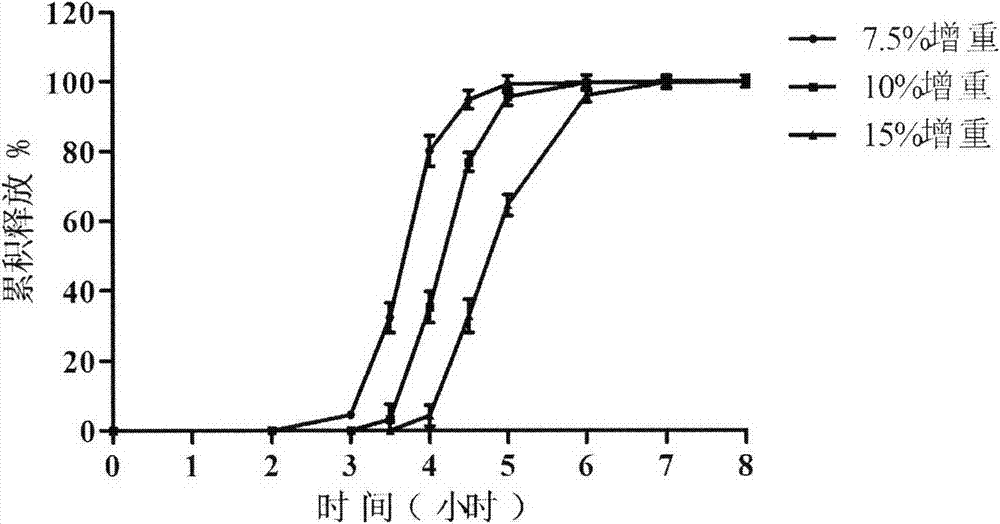
[0045] A. Preparation of micronized medicine: take 55g zolpidem tartrate raw material, put in jet mill, set powder delivery pressure as 4bar, grinding pressure 2bar, powder delivery speed 0.5g / min, pulverization time is 30min, particle is carried out Micronized pulverization, continuous pulverization twice to obtain micronized drug (average particle size 0.5-3 μm), the yield is 91%;
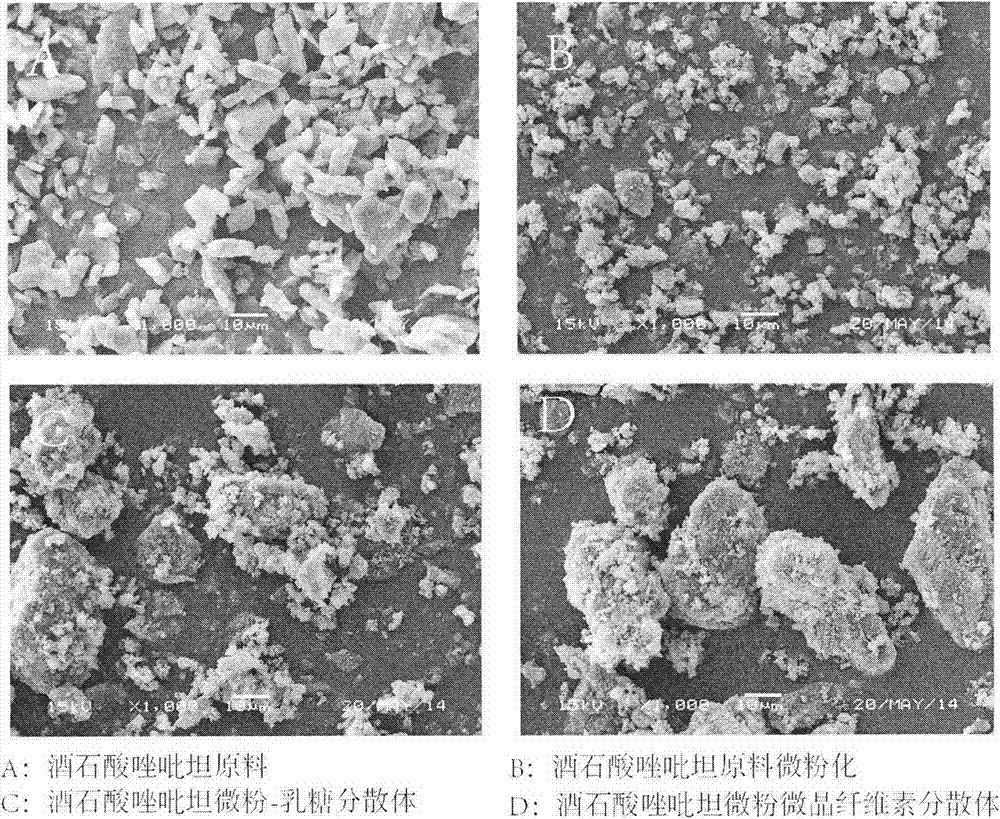
[0046] B. the preparation of submicron solid dispersion medicine: take by weighing 50g micronized medicine, and 290g solid dispersion carrier lactose ( 200) Place a cube mixer at a rotational speed of 180rpm, and mix for 10 minutes; take the solid dispersion mixed drug and put it in a small ball mill, add zirconia balls, and grind for 2 hours to obtain a submicron solid dispersed drug. Its scanning electron microscope picture is shown in figure 1 .
[004...
Embodiment 2
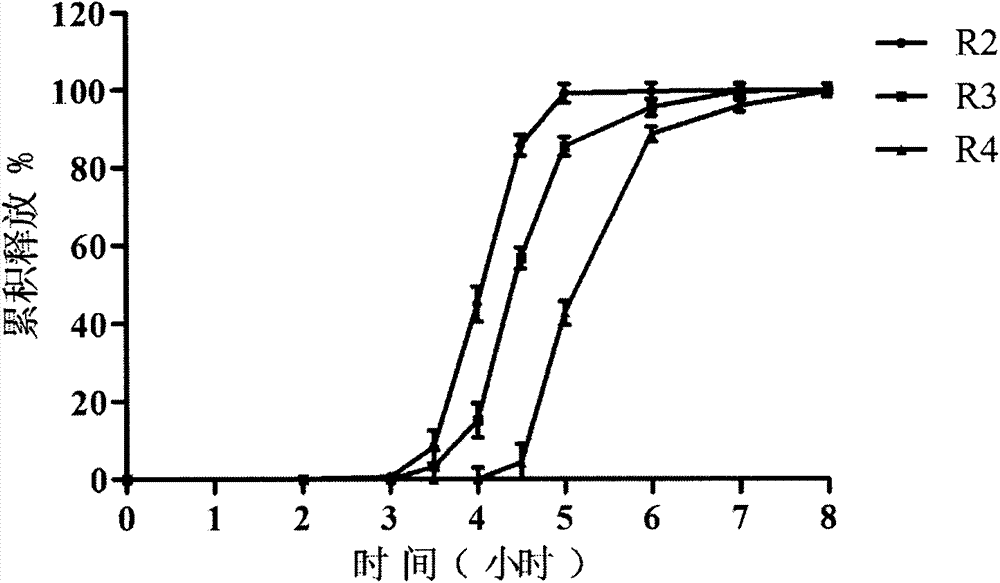
[0055] R 2 Tablet prescription:
[0056]
[0057] Preparation Process:
[0058] (1) Preparation of Submicron Solid Dispersion Drugs
[0059] A. Preparation of micronized medicine: take 53g zolpidem tartrate raw material, put in the jet mill, set the powder conveying pressure as 5bar, grinding pressure 3bar, powder conveying speed 0.6g / min, crushing time is 35min, the particle is carried out Micronized pulverization, continuous pulverization twice to obtain micronized drug (average particle size 0.2-5 μm), the yield is 94%;
[0060] B. Preparation of submicron solid dispersion drug: Weigh 50g of micronized drug, put it in a cube mixer with 250g of solid dispersion carrier compressible starch, and mix it for 15min at a speed of 150rpm; take the solid dispersion mixed drug and put it in a small ball mill, add Zirconia grinding balls, grind for 3 hours to obtain submicron solid-dispersed drugs.
[0061] (2) Preparation of drug-containing tablet core in solubilization system...
Embodiment 3
[0069] R 3 Tablet prescription:
[0070]
[0071] Preparation Process:
[0072] (1) Preparation of Submicron Solid Dispersion Drugs
[0073] Preparation of micronized drug: Weigh 54g zolpidem tartrate raw material, put it in the jet mill, set the powder delivery pressure to 6bar, the grinding pressure to 3bar, the powder delivery speed to 0.3g / min, and the pulverization time to 35min, and the particles are micronized Pulverize, pulverize twice in a row to obtain micronized drug (average particle size 0.5-8 μm), yield is 92.5%;
[0074] Preparation of submicron solid-dispersed drug: Weigh an appropriate amount of micronized drug, put it in a cube mixer with solid dispersion carrier lactose (Tablettose80), and mix it for 20 minutes at a speed of 150 rpm; take the solid-dispersed mixed drug and put it in a small ball mill, add zirconia mill balls, and grind for 4 hours to obtain submicron solid-dispersed drugs.
[0075] (2) Preparation of solubilizing system drug-containin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com