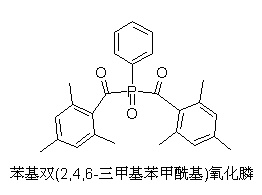
Preparation method of phenyl bis(2,4,6-trimethylbenzoyl)phosphine oxide
A technology of trimethylbenzoyl and phenylphosphine oxide, which is applied in the field of preparation of high-efficiency free radical photoinitiator phenylphosphine oxide, can solve the problems of high cost, difficult industrial implementation, and large amount of waste water, and achieve cost saving , avoid the effect of high price and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide
[0034] 1) Preparation of phenylphosphine:
[0035] Add 800ml of toluene and sodium block (10.1g, 0.88mol) into a 2000ml reaction flask under nitrogen protection, heat it to reflux, stir vigorously until the sodium block becomes very fine, slowly add P,P- Dichlorophenylphosphine (39.4g, 0.22mol) was dripped in 0.5h, the reaction was continued for 16h, the temperature was lowered to below the boiling point, water (10.0g, 0.55mol) was added dropwise within 0.5h, and the reaction was refluxed until the sodium was completely consumed to obtain Phenylphosphine-containing solution. The solvent was recovered by low-pressure distillation, and the fractions at 40-45°C / 10 mHg were collected and confirmed by nuclear magnetic resonance. 31 P: -123ppm. After the solvent is recovered, the next step reaction can also be carried out directly without purification;
[0036] 2) Preparation of phenylphosphine o...
Embodiment 2
[0042] Example 2: Preparation of phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide
[0043] 1) Bishydroxyphosphine oxide ( A ) preparation
[0044] Under nitrogen protection, dissolve 12.6g of phenylphosphine oxide (0.10mol) in 100ml of dichloroethane, add 40.0g of 20% sodium hydroxide, cool down to -5°C, and slowly dropwise add 37.1g of 2,4,6 -Trimethylbenzaldehyde (0.25mol), dripped for 1h, then heated up for reaction, heated to 60°C, kept for reaction, 31 P-NMR monitors the reaction, when the phenylphosphine oxide disappears, the reaction is complete, and the reaction is lowered to room temperature, and the obtained reaction solution can be directly reacted in the next step without purification;
[0045] 2) Preparation of phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide
[0046] The bishydroxyphosphine oxide prepared in step 3) ( A ) The reaction solution was directly used in this step, and 2.5g of V(IV)(acac) was added. 2 , under stirring, slowly add 28.3g of 30% h...
Embodiment 3
[0047] Example 3: Preparation of phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide
[0048] 1) Bishydroxyphosphine oxide ( A ) preparation
[0049] Under nitrogen protection, 12.6g of phenylphosphine oxide (0.10mol) was dissolved in 100ml of dichloroethane, 22.5g of 40% dimethylamine aqueous solution was added respectively, the temperature was lowered to -5°C, and 37.1g of 2,4 was slowly added dropwise. , 6-trimethylbenzaldehyde (0.25mol), dripped for 1h, then heated up for the reaction, heated to 60°C, kept for the reaction, 31 P-NMR monitors the reaction, when the phenylphosphine oxide disappears, the reaction is complete, and the reaction is lowered to room temperature, and the obtained reaction solution can be directly reacted in the next step without purification;
[0050] 2) Preparation of phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide
[0051] The bishydroxyphosphine oxide prepared in step 3) ( A ) The reaction solution was directly used in this step, and 2.5g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com