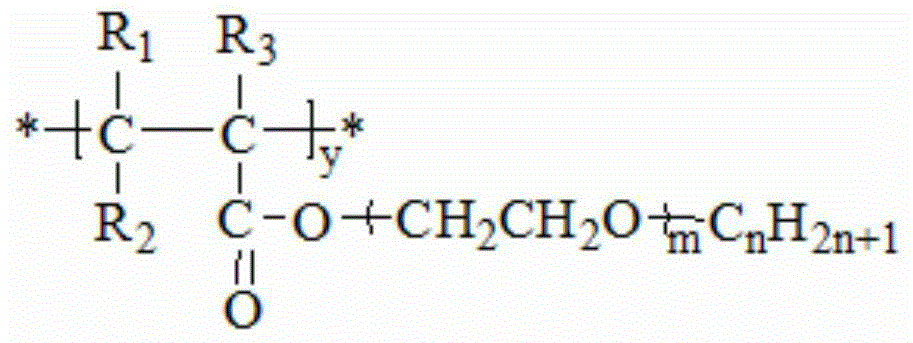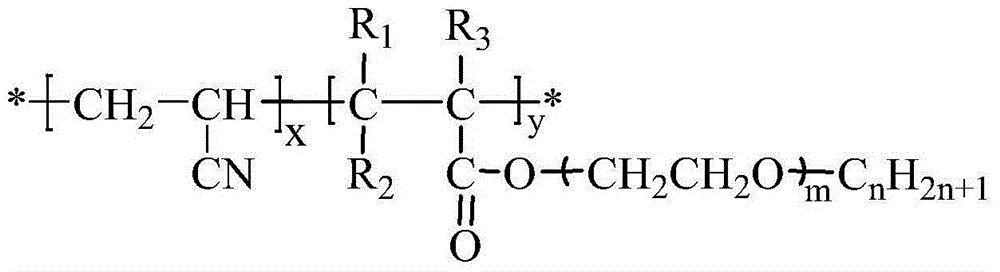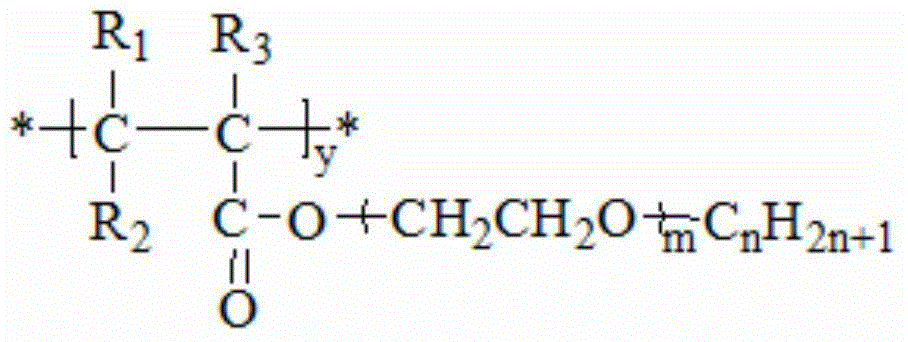Second monomer of acrylonitrile copolymer solid-solid phase change material and preparation method and use of second monomer
A technology of acrylonitrile-based copolymer and phase change material, which can be used in the preparation of ester group and hydroxyl group, heat exchange materials, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] ①Preparation of the second monomer of acrylonitrile-based copolymer solid-solid phase change material
[0068] The material selected in this embodiment and its content are:
[0069] Polyethylene glycol alkyl ether: polyethylene glycol 100 (HO (CH 2 CH 2 O) 100 H);
[0070] Ester monomers containing unsaturated double bonds: ethyl acrylate monomer;
[0071] The molar ratio of polyethylene glycol 100 and ethyl acrylate monomer is 85:15;
[0072] Catalyst: sodium methyl, the consumption is 9% of the mass sum of polyethylene glycol 100 and ethyl acrylate monomer;
[0073] Polymerization inhibitor: cuprous chloride, the dosage is 1% of the mass sum of polyethylene glycol 100 and ethyl acrylate monomer;
[0074] The preparation method is as follows:
[0075] Add methyl sodium, cuprous chloride and polyethylene glycol 100 (HO(CH 2 CH 2 O) 100 H), stirring while raising the system temperature to 30°C, all the reactants melted, and then heating to make the system tempe...
Embodiment 2
[0083] ①Preparation of the second monomer of acrylonitrile-based copolymer solid-solid phase change material
[0084] Polyethylene glycol 100 in embodiment 1 is replaced by diethylene glycol cetyl ether (HO(CH 2 CH 2 O) 2 C 16 h 33 ); ethyl acrylate monomer is replaced by propyl acrylate; catalyst sodium methyl is replaced by benzenesulfonic acid, and consumption is 2% of diethylene glycol cetyl ether and propyl acrylate quality and; Copper is replaced by hydroquinone, and the dosage is 1% of the mass sum of diethylene glycol cetyl ether and propyl acrylate, and the second monomer diethylene glycol cetyl ether monoacrylate is prepared in the same way , the yield was 76%.
[0085] ②Preparation of acrylonitrile-based copolymer phase change materials by solution polymerization
[0086] The solvent N'N'-dimethylformamide in the implementation 1 is replaced by dimethyl sulfoxide (the reaction monomer concentration is 50wt%), and the reaction monomer is acrylonitrile and The ...
Embodiment 3
[0092] ①Preparation of the second monomer of acrylonitrile-based copolymer solid-solid phase change material
[0093] Polyethylene glycol 100 in embodiment 1 is replaced by polyethylene glycol behenyl ether (HO(CH 2 CH 2 O) 100 C 22 h 45 ); the ethyl acrylate monomer is replaced by n-propyl methacrylate; the second monomer polyethylene glycol behenyl ether mono-n-propyl methacrylate is prepared in the same way, wherein n-propyl methacrylate drops After the addition, the temperature of the system was controlled at 90° C., and the reaction was carried out for 18 hours, and the yield was 87%.
[0094] ②Preparation of acrylonitrile-based copolymer phase change materials by solution polymerization
[0095] The solvent N'N'-dimethylformamide in Example 1 is replaced by N-methylpyrrolidone (the reaction monomer concentration is 30wt%), and the reaction monomer is acrylonitrile with a uniform molar ratio of 95:5 and the second monomer polyethylene glycol behenyl ether mono-n-pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com