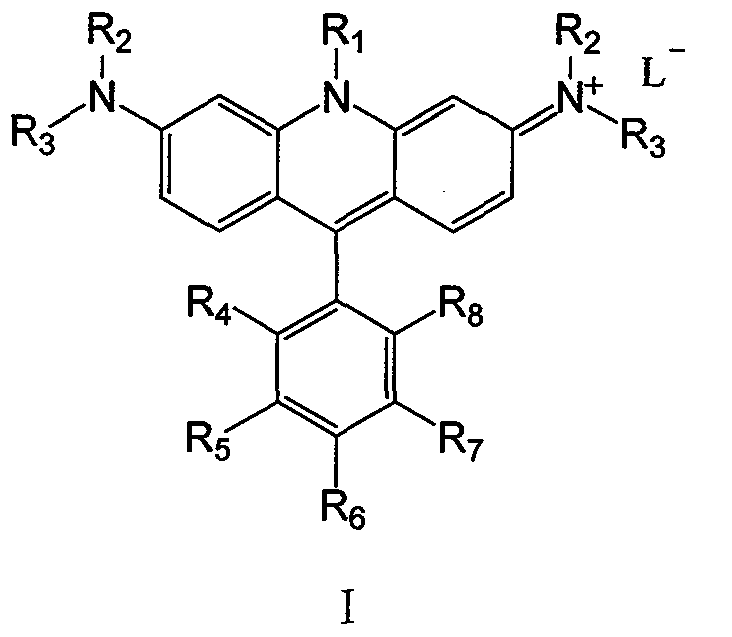
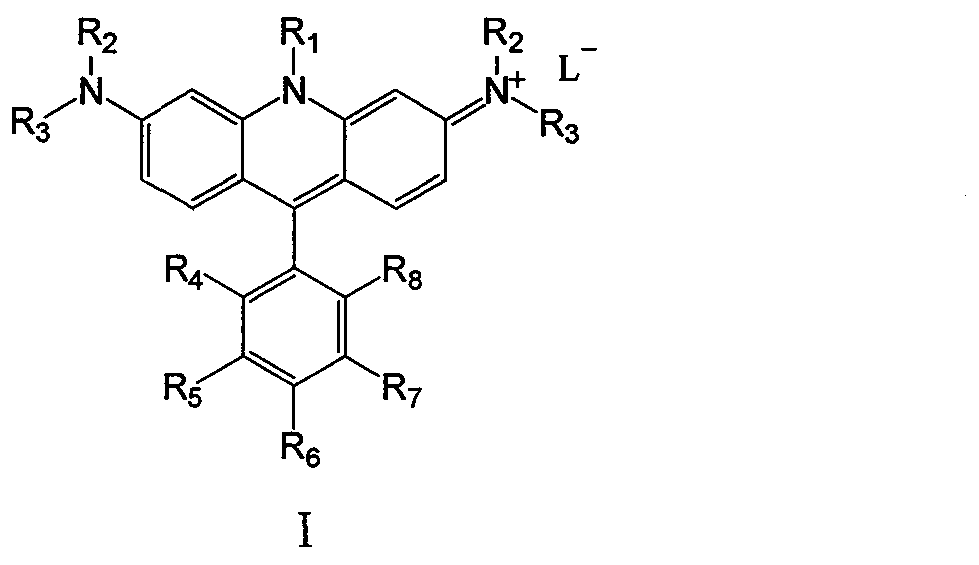
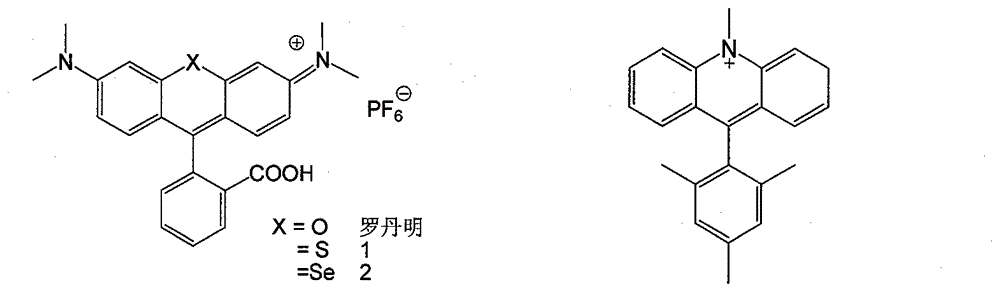
Nitrogen-containing cation chromophore and preparation thereof
A technology of cationic and nitrogen elements, applied in the direction of luminescent materials, acridine dyes, organic dyes, etc., can solve the problems of poor solubility, catalyst deactivation, unstable reaction system, etc., to prevent accumulation effect, improve stability, and save time long effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0035]
[0036] Step ①: Put 0.04 mol of N,N-dimethyl-m-bromoaniline, 21.6mL of 30% methylamine aqueous solution (containing about 0.10 mol of methylamine), and 0.002 mol of copper powder into a 50mL sealed tube, and react at 100°C for 12h Afterwards, cool to room temperature, extract 3 times with 40 mL of ethyl acetate, combine the organic phases, dry with anhydrous sodium sulfate, filter, concentrate, recrystallize in ethanol for separation and purification, and obtain 5.64 g of the reaction product of step ①;
[0037] The second step: 0.024 moles of the first step product, 0.020 moles of N,N-dimethyl-iodoaniline and 0.024 moles of KN(Si(CH 3 ) 3 ) 2 Dissolve in 50 mL of dioxane, under nitrogen protection, react at 100°C for 30 minutes, add 15 mL of water to quench the reaction, cool to room temperature, extract 3 times with 30 mL of ethyl acetate / tetrahydrofuran with a molar ratio of 1:1, and combine The organic phase was saturated with Na 2 CO 3 The solution was wash...
example 2
[0040]
[0041] Step ①: Put 0.02 mol of N,N-dimethyl-m-bromoaniline, 0.04 mol of benzylamine, and 0.01 mol of copper powder into a 25mL sealed tube, react at 100°C for 12h, cool to room temperature, and wash with 30mL ethyl acetate The ester was extracted 3 times, the organic phases were combined, dried with anhydrous sodium sulfate, filtered, concentrated, separated and purified by recrystallization in ethanol to obtain 4.10 g of the reaction product of step ①;
[0042] The ② step: 0.018 moles of the ① step product, 0.015 moles of N, N-dimethyl iodoaniline and 0.018 moles of KN(Si(CH 3 ) 3 ) 2 Dissolve in 30mL of dioxane, under the protection of nitrogen, react at 100°C for 30 minutes, add 10mL of water to quench the reaction, cool to room temperature, extract 3 times with 20mL of ethyl acetate / tetrahydrofuran with a molar ratio of 1:1, and combine The organic phase was saturated with Na 2 CO 3 The solution was washed, dried over anhydrous sodium sulfate, filtered, con...
example 3
[0045]
[0046] Step ①: put 1.6 moles of N, N-dimethyl-m-bromoaniline, 800 mL of 30% methylamine aqueous solution (containing about 4 moles of methylamine), and 0.08 moles of copper powder into a 1.5 L sealed tube, and react at 100 ° C for 18 hours Afterwards, cool to room temperature, extract 3 times with 300 mL ethyl acetate, combine the organic phases, dry with anhydrous sodium sulfate, filter, concentrate, separate and purify by recrystallization in ethanol, and obtain 226.30 g of the reaction product of step ①;
[0047] Step ②: 1.2 moles of the product of step ①, 1 mole of N,N-dimethyl-iodoaniline and 1.2 moles of KN(Si(CH 3 ) 3 ) 2 Dissolve in 1.5L of dioxane, react at 100°C for 50 minutes under nitrogen protection, add 2.5L of water to quench the reaction, cool to room temperature, and extract 3 times with 120mL of ethyl acetate / tetrahydrofuran with a molar ratio of 1:1 , combine the organic phases and wash with saturated Na 2 CO 3 The solution was washed, dried ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com