Aromatic amine derivative, and preparation method, use and organic electroluminescent device thereof
A technology of aromatic amine derivatives and triarylamine, which is applied in the field of organic electroluminescence, can solve the problems of low lifetime of organic electroluminescent devices, unfavorable preparation of organic electroluminescent devices, and insufficient stability of redox repeatability, etc., and achieves good results. Film-forming properties, large molecular weight, and good service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
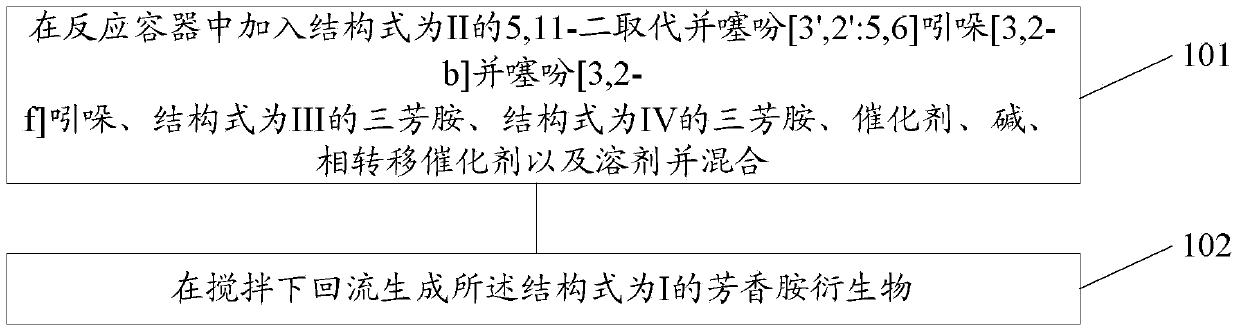
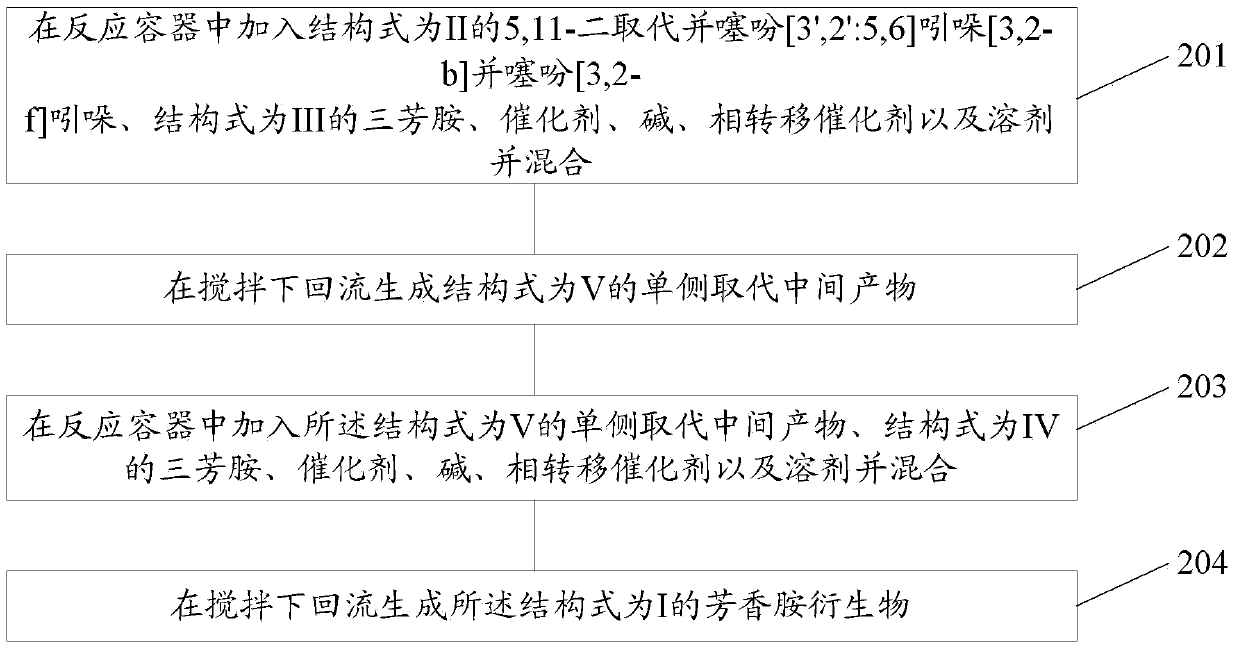
[0058] Corresponding to the above-mentioned aromatic amine derivatives, the present invention also provides its preparation method, such as figure 1 shown, including:
[0059] 101. Add 5,11-disubstituted thiophene[3',2':5,6]indole[3,2-b]thiophene[3,2-f]indole with structural formula II into the reaction vessel , a triarylamine with a structural formula III, a triarylamine with a structural formula IV, a catalyst, a base, a phase transfer catalyst and a solvent and mix them.
[0060]
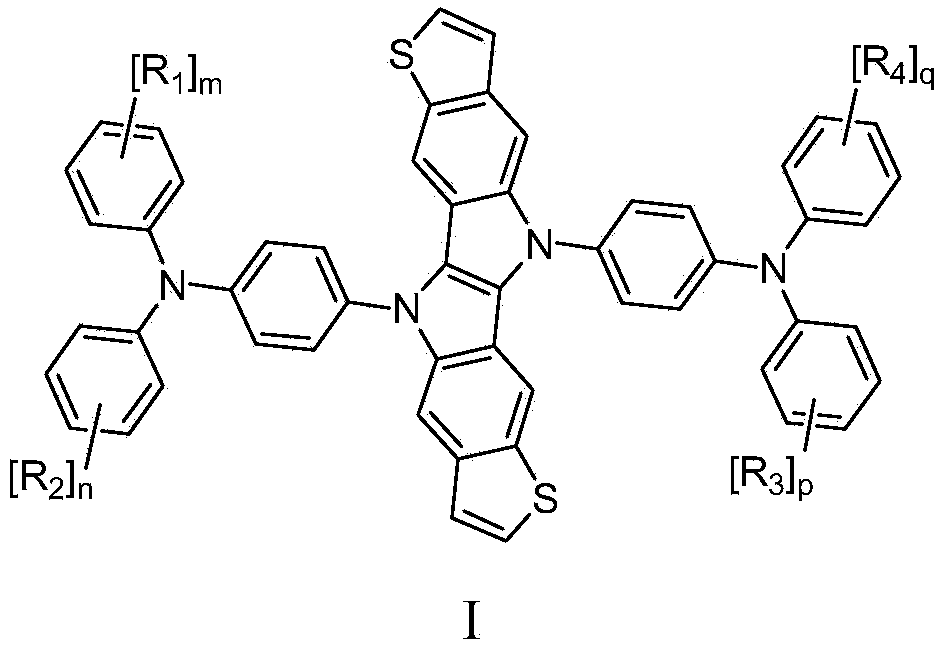
[0061] X and Y in the triarylamines III and IV each independently represent a halogen, such as F, Cl, Br, I and the like.
[0062] The 5,11-disubstituted thiophene[3',2':5,6]indole[3,2-b]thiophene[3,2-f]indole in this example can be obtained through literature Chem.Commun ., 2012, 48, 12225-12227 records the method to prepare, and will not repeat them here.
[0063] The triarylamines with the structural formula III and the triarylamines with the structural formula IV can be obtained through...
Embodiment 1
[0181] ITO / I-1(40nm) / CBP:6%(Firpic30nm) / Bphen(25nm) / LiF(0.5nm) / Al(100nm)
Embodiment 2
[0183] ITO / I-9(40nm) / CBP:6%Firpic(30nm) / Bphen(25nm) / LiF(0.5nm) / Al(100nm)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com