Transformation system for Spirodelapolyrrhiza callus induction and capable of realizing transgene stable inheritance
A transgenic and stable technology, which is applied in the field of Ziping callus to obtain stable genetic regenerated plants, can solve the problems of poor growth and achieve high-efficiency expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
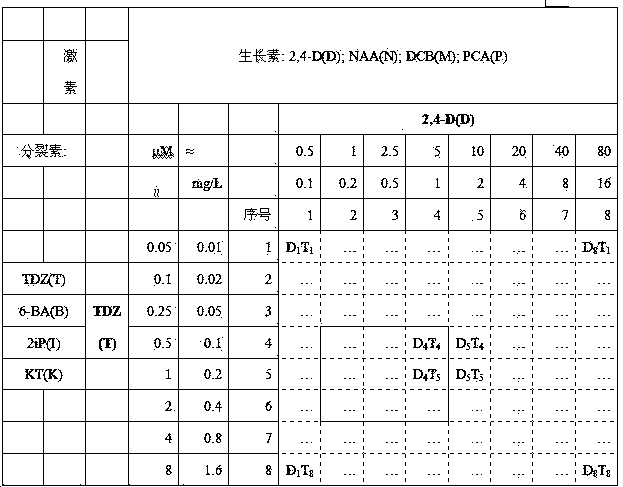
[0057] Screening of different phytohormone combinations and concentrations:
[0058] Each phytohormone combination contains an auxin and a plant cytokinin.
[0059] Plant auxins include: 2,4-D, 2,4-Dichlorophenoxyacetic Acid (2,4-D, 2,4-Dichlorophenoxyacetic Acid), 1-Naphthylacetic Acid (NAA, 1-Naphthylacetic Acid), Dicamba (DCB, Dicamba) and p-Chlorophenoxy acetic acid (PCA, p-Chlorophenoxy acetic acid ).
[0060] Plant cytokinins include: thiadizuron (TDZ, thidiazuron), 6-benzylaminopurine (6-BA, 6-Benzylaminopurine), N 6 -Isopentenyl adenine (2iP, N 6 -(2-Isopentenyl) adenine) and kinetin (KT, kineting).
[0061] The phytohormone combinations and their concentrations are shown in the table below. Eight concentrations of each phytohormone were tested. such as D 1 T 8 represents the combination of 2,4-D (0.1 mg / L) and TDZ (1.6 mg / L), M 5 I 4 Represents the combination of DCB (2 mg / L) and 2iP (0.1 mg / L), and so on.
[0062] The front (adaxial face, adaxial face) of ...
Embodiment 2
[0067] Effects of different media on callus induction of Ziping:
[0068] In order to test whether different media can affect the callus induction of Ziping, the following six media were tested: MS (Murashige and Skoog), N6 (Zhu Zhiqing), B5 (Gamborg B5), WP (McCown Woody Plant), SH (Schenk and Hildebrandt), NN (Nitsch JP and Nitsch C) media. Prepare 50 ml of each culture medium (containing 3% sucrose, 0.7% Agar, D 4 T 6 , pH 5.8), sterilized at 121°C for 20 minutes, and evenly divided into two glass Petri dishes with a diameter of 9 cm. Put 6 clumps of rooted Zipain fronds on each petri dish, the front side (adaxial face, adaxial face) of Zihuai fronds is in contact with the callus induction medium, and the back side (off-axis face, abaxial face) is upward, and the pseudo The roots were suspended in the air and cultured in the dark at 25°C for 2 weeks. The 6 culture media can induce callus to varying degrees, and the callus induction rate ranges from 30% to 100%. Relativ...
Embodiment 3
[0070] Effects of different light conditions on callus induction of Ziping
[0071] In order to test whether different light conditions have an effect on the callus induction of Ziping, three light conditions were tested: total darkness,
[0072] Semi-dark, illuminated.
[0073] Total darkness: put the petri dish in an airtight carton and put it in an illumination of 2000 Lux (about 25 μmolm -2 the s -1 ) plant lighting incubator, the photoperiod is 16h light / 8h dark.
[0074] Semi-darkness: Place the petri dish in a carton with an open top, partially cover the top of the carton with white thin paper so that the illuminance inside the carton is around 400 Lux (about 5 μmolm -2 the s -1 ). The light intensity of the plant light incubator is 2000 Lux, and the light cycle is 16h light / 8h dark.
[0075] Illumination: Illumination is 2000 Lux, and the photoperiod is 16h light / 8h dark.
[0076] Prepare 300 ml of MS medium (containing 3% sucrose, 0.7% Agar, D 4 T 6 , pH 5.8)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com