Composition for skin containing silicone base
A composition and skin technology, which can be applied in the directions of medical preparations containing active ingredients, medical preparations without active ingredients, skin diseases, etc., can solve the problems of deterioration, increase, and damage to the feeling of touch and use of the composition, etc., To achieve the effect of excellent touch and use, excellent use, and high mixing ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
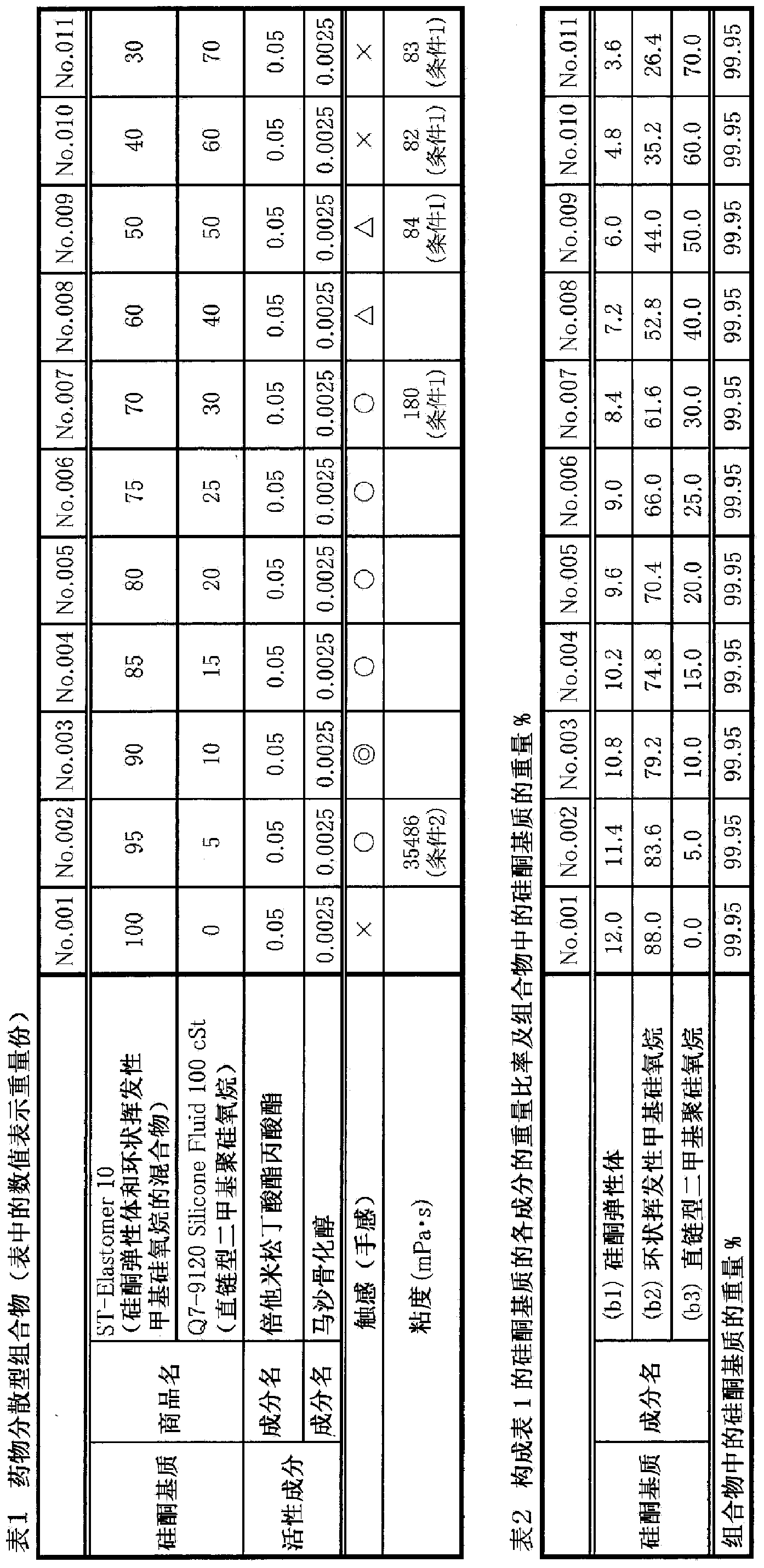
[0059] [Example 1] Preparation and evaluation of drug-dispersed composition
[0060] Compositions having the compositions shown in Tables 1 and 2 were prepared in the following procedure. In addition, as active ingredients, steroid (betamethasone butyrate propionate) and active VD 3 (Maxacalcitol). In addition, as a mixture of the silicone elastomer (b1) and the cyclic volatile methylsiloxane [decamethylcyclopentasiloxane] (b2), ST-Elastomer 10 (silicone from DOW CORNING) was used. Elastomer: decamethylcyclopentasiloxane = 12:88 [weight ratio]), and as the linear type dimethyl polysiloxane (b3), Q7-9120 Silicone Fluid 100CST of Dow Corning Corporation was used.
[0061] Preparation method of drug dispersion type composition
[0062] (1) Weigh the mixture of (b1) and (b2) in a suitable container.
[0063] (2) Add (b3) to (1) and mix.
[0064] (3) The active ingredient was added to (2), and stirred with a homomixer (6000 rpm, 5 minutes) at room temperature to disperse it. ...
Embodiment 2
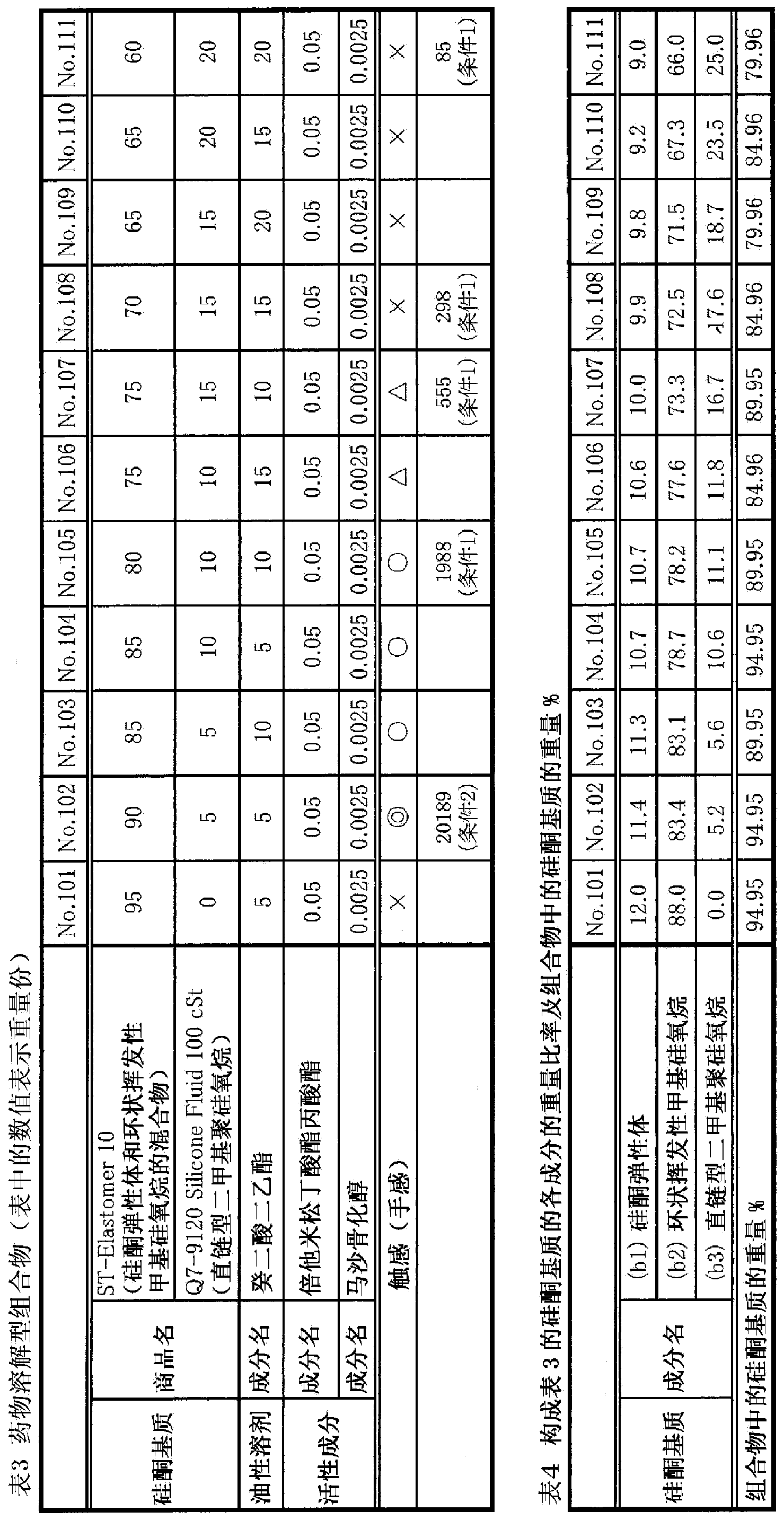
[0072] [Example 2] Preparation of drug-dissolving composition
[0073] Compositions having the compositions shown in Tables 3 and 4 were prepared in the following procedure. In addition, the mixture of active ingredient, silicone elastomer (b1) and cyclic volatile methylsiloxane [decamethylcyclopentasiloxane] (b2), linear dimethylpolysiloxane (b3 ) The same thing as in Example 1 was used, and Nikkol DES-SP of Nikko Kagaku was used as diethyl sebacate.
[0074] Preparation method of drug-dissolving composition
[0075] (1) Weigh the mixture of (b1) and (b2) in a suitable container.
[0076] (2) Add (b3) to (1) and mix.
[0077] (3) The active ingredient previously dissolved in the oily solvent was added to (2), and it stirred and mixed with the homomixer (6000 rpm, 5 minutes) at room temperature.
[0078]The composition prepared in the above-mentioned manner is a white translucent gel-liquid composition. When each composition was observed with a microscope, no crystal disp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com