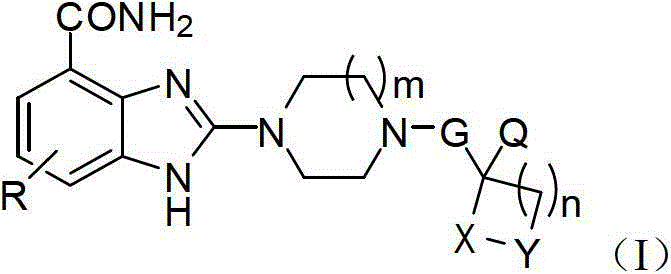
Benzimidazole-2-piperazine compound, pharmaceutical composition of compound and preparing method and application of pharmaceutical composition
A technology of benzimidazoles and compounds, applied in the field of poly(ADP-ribose) polymerase (PARP) inhibitors, can solve problems such as inability to perform repair process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
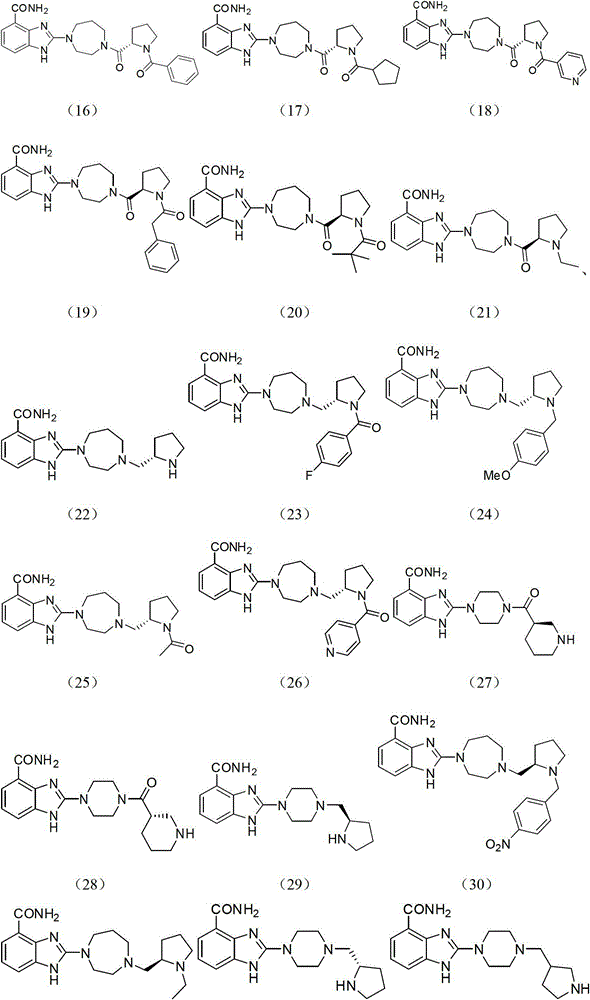
Image
Examples
Embodiment 1
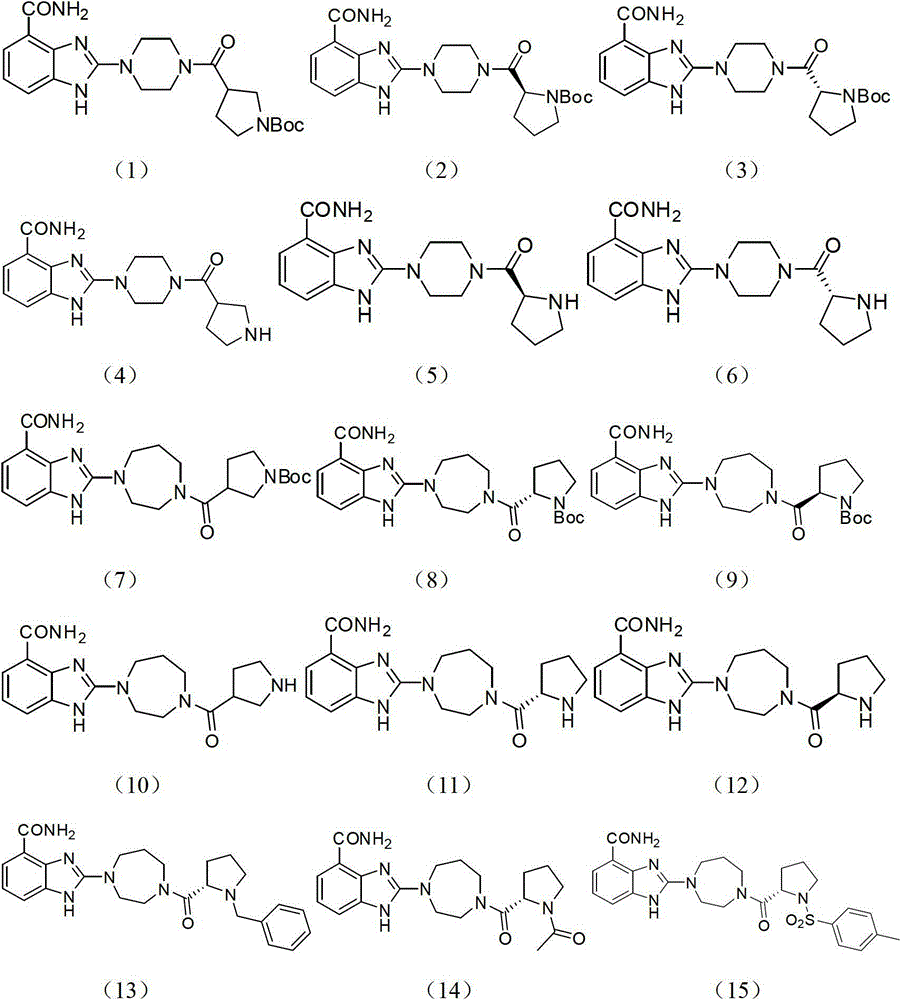
[0104] Compound (1): 3-(4-(4-carbamoyl-1hydro-benzimidazol-2-yl)piperazine-1-carbonyl)pyrrolidine-1-tert-butyl carbonate, the reaction formula is as follows:
[0105]
[0106] Step 1: Compound a: 2-oxo-2,3-dihydro-1hydro-benzimidazole-4-carboxylic acid methyl ester
[0107] Add carbonyl diimidazole (1.56g, 9.6mmol) to an anhydrous tetrahydrofuran solution (20mL) with methyl 2,3-diaminobenzoate (0.8g, 4.8mmol) dissolved, heat to reflux, and react for 8 hours After cooling, the solvent was removed under reduced pressure, and the residue was separated by flash column chromatography (petroleum ether: ethyl acetate = 5:1) to obtain a pale yellow solid compound a (0.3 g, yield 33%). MS(ESI)m / z:[M+H] + =193.
[0108] Step 2: Compound b: 2-chloro-1hydro-benzimidazole-4-carboxylic acid methyl ester
[0109] Compound a (1.1g, 5.7mmol) was added to phosphorus oxychloride (8mL), heated to reflux, reacted for 8 hours and then cooled, the solvent was removed under reduced pressure, and the residue...
Embodiment 2
[0117] Compound (2): (S)-2-(4-(4-carbamoyl-1hydro-benzimidazol-2-yl)piperazine-1-carbonyl)pyrrolidine-1-tert-butyl carbonate
[0118]
[0119] A method similar to the preparation of compound (1) in Example 1 was adopted, that is, compound (2) (21 mg, yield 11%) was obtained by condensation of compound d with (S)-1-tert-butoxycarbonylpyrrolidine-2-carboxylic acid. MS(ESI)m / z:[M+H] + =443. 1 H NMR (300MHz, DMSO-d6): δ11.99(br,1H), 9.10(br,1H), 7.61(d,1H,J=7.5Hz), 7.51(br,1H), 7.33(d,1H) ,J=7.5Hz),6.98(t,1H,J=7.5Hz),4.70-4.63(m,1H),3.70-3.56(m,10H),2.19-2.11(m,2H),1.79-1.73( m, 2H), 1.36 (s, 9H).
Embodiment 3
[0121] Compound (3): (R)-2-(4-(4-carbamoyl-1hydro-benzimidazol-2-yl)piperazine-1-carbonyl)pyrrolidine-1-tert-butyl carbonate
[0122]
[0123] Using a method similar to the preparation of compound (1) in Example 1, compound (3) (18 mg, yield 10%) was obtained by condensation of compound d and (R)-1-tert-butoxycarbonylpyrrolidine-2-carboxylic acid. MS(ESI)m / z:[M+H] + =443. 1 H NMR (300MHz, DMSO-d6): δ11.84(br,1H), 9.10(br,1H), 7.61(d,1H,J=7.5Hz), 7.51(br,1H), 7.32(d,1H) ,J=7.5Hz),6.99(t,1H,J=7.5Hz),4.69-4.65(m,1H),3.70-3.55(m,10H),2.21-2.09(m,2H),1.79-1.74( m, 2H), 1.29 (s, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com