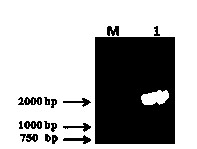
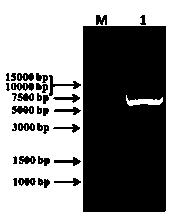
Method for expressing and purifying human cytoplasmic gelsolin
A gelsolin, expression and purification technology, applied in the direction of animal/human peptides, the use of vectors to introduce foreign genetic material, recombinant DNA technology, etc., can solve the problems of low expression of cytoplasmic gelsolin and difficult purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The primers for amplifying human cytoplasmic gelsolin hcGSN gene were synthesized, and the primers were designed as follows:
[0023] Upstream primer FW (hcGSN): 5'- CAGGCCTCGAGGTGGTGGAACACCCCCGAGTTCCTC -3';
[0024] Downstream primer RV (hcGSN): 5'-CACGCCTCGAGCTAGGCAGCCAGCTCAGCCATGG-3';
[0025] Restriction endonuclease introduced in both upstream and downstream primers xho The single enzyme cutting site of I.
Embodiment 2
[0027] This example illustrates the construction of pET-15b-hcGSN recombinant expression vector.
[0028] Using human plasma gelsolin cDNA (GenBank: X04412.1) as a template, using the upstream primer FW (hcGSN) and downstream primer RV (hcGSN) in Example 1, the hcGSN gene was amplified by PCR. The specific PCR program is as follows: pre-denaturation at 95°C for 5 minutes; denaturation at 95°C for 1 minute, annealing at 64°C for 1 minute, extension at 72°C for 2 minutes, the total number of cycles is 25; and finally extension at 72°C for 10 minutes. The PCR product and the prokaryotic expression vector pET15b were respectively digested with restriction endonuclease XhoI. The digested products were subjected to 1% agarose gel electrophoresis, and the corresponding DNA fragments were recovered with a gel recovery kit. T4 DNA ligase was used for overnight ligation at 16°C, and the ligation product was transformed into Escherichia coli DH5α competent cells and cultured at 37°C. S...
Embodiment 3
[0030] This example illustrates the expression of hcGSN recombinant protein.
[0031] The pET-15b-hcGSN recombinant expression plasmid constructed in Example 2 was transformed into Escherichia coli BL21 (DE3) competent cells to obtain an engineering strain containing the recombinant plasmid. A single colony of the engineered strain was picked, inoculated in LB liquid medium, and cultured overnight at 37°C. The next day, transfer to fresh LB medium according to 1% inoculum volume, and shake culture at 37°C until OD 600nm After reaching 0.6-0.8, add IPTG with a final concentration of 0.4 for induction for 4 hours. At the same time, the bacterial solution induced without IPTG was set as a control. Then the bacterial liquid was collected and centrifuged at 5000rpm to obtain bacterial pellet. Add SDS-PAGE gel electrophoresis loading buffer 50mM Tris-HCl (containing 2% SDS, 0.1% bromophenol blue, 10% glycerol, 50 mM NaCl, 0.5 mM PMSF, 100mM DTT, pH 6.8) to the pellet, Lyse E. co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com