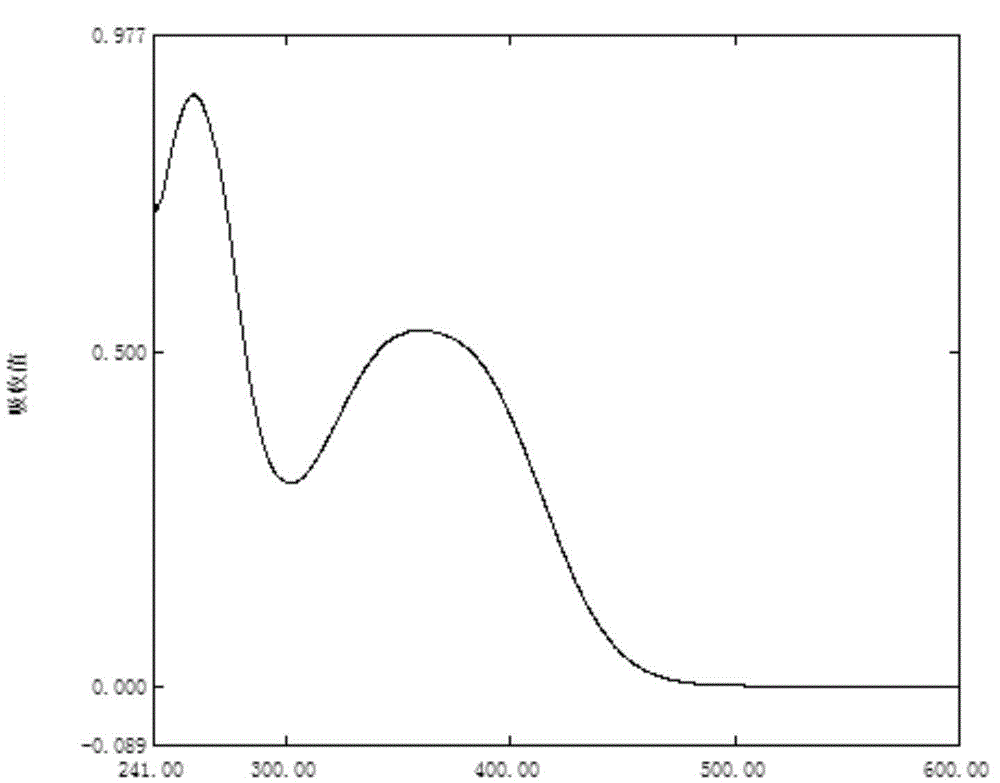
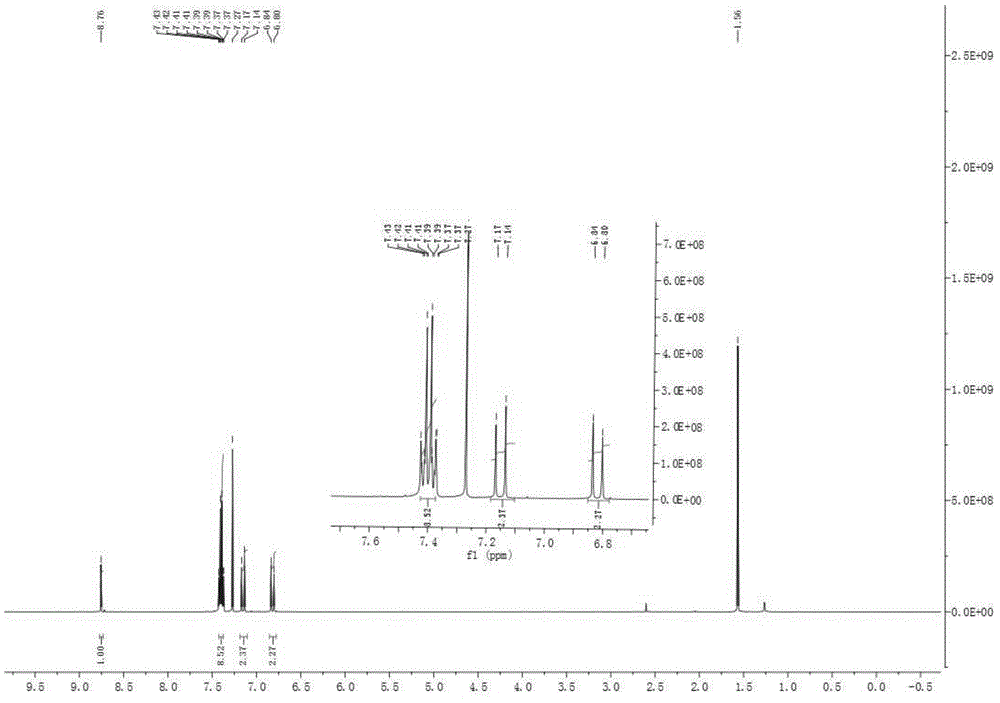
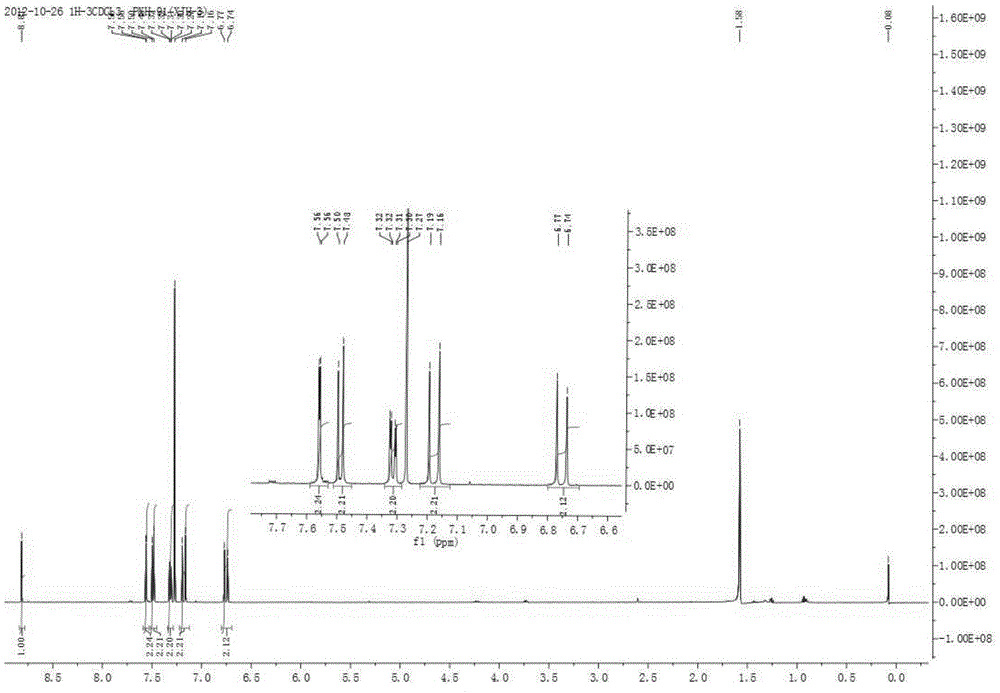
Chlorinated 2, 4, 6-trinitro-1, 3- distyryl benzene derivatives as well as preparation method and application thereof
A technology of distyrylbenzene and trinitro, which is applied in the preparation of organic compounds, aromatic nitration compositions, organic chemistry, etc., can solve the problems of difficult temperature control and compound decomposition, and achieve wide absorption band and good optics The effect of the characteristic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 2,4,6-Trinitro-1,3-bis(4’-chlorostyryl)benzene (R 1 , R 2 is a hydrogen atom, R 3 for chlorine atom) microwave synthesis:
[0027] Mix and dissolve 0.48g (2.0mmol) of 2,4,6-trinitro-m-xylene and 0.59g (4.2mmol) of p-chlorobenzaldehyde in 20mL of dichloromethane, add dropwise 0.08g (0.9mmol) of piperidine and Carrier silica gel (3.0g), the raw material and basic catalyst are evenly loaded on the carrier, and the solvent is removed under normal pressure to obtain a solid mixture of loaded raw materials. After mixing uniformly, the obtained solid is transferred to a Pyrex reaction bottle and exposed to microwave In the reactor, take it out after 15 minutes of microwave under 300W power. After the reaction is finished, the product is extracted with acetone, and then the acetone is removed to obtain a crude product, which is finally recrystallized with a mixed solvent of acetone and ethanol. The title compound (0.75 g) was obtained in a yield of 77%.
[0028] Yellow cry...
Embodiment 2
[0033] 2,4,6-Trinitro-1,3-bis(2’-chlorostyryl)benzene (R 2 , R 3 is a hydrogen atom, R 1 for chlorine atom) microwave synthesis:
[0034] Mix and dissolve 0.48g (2.0mmol) of 2,4,6-trinitro-m-xylene and 0.59g (4.2mmol) of o-chlorobenzaldehyde in 20mL of dichloromethane, add dropwise 0.08g (0.9mmol) of piperidine and The carrier NaOH (3.0g), the raw material and the basic catalyst are evenly loaded on the carrier, and the solvent is removed under normal pressure to obtain a solid mixture of loaded raw materials. After mixing evenly, the obtained solid is transferred to a Pyrex reaction bottle and placed in a microwave oven. In the reactor, take it out after 15 minutes of microwave under 300W power. After the reaction is finished, the product is extracted with acetone, and then the acetone is removed to obtain a crude product, which is finally recrystallized with a mixed solvent of acetone and ethanol. The title compound (0.88 g) was obtained with a yield of 85.7%.
[0035] ...
Embodiment 3
[0040] 2,4,6-Trinitro-1,3-bis(2’,4’-dichlorostyryl)benzene (R 1 , R 3 is chlorine, R 2 for the microwave synthesis of hydrogen atoms):
[0041] Mix and dissolve 0.48g (2.0mmol) of 2,4,6-trinitro-m-xylene and 2,4-dichlorobenzaldehyde (0.70g, 4.2mmol) in 20mL of dichloromethane, add dropwise morpholine and carrier Potassium carbonate (3.0g), the raw material and the basic catalyst are evenly loaded on the carrier, and the solvent is removed under normal pressure to obtain a solid mixture of loaded raw materials. After mixing uniformly, the obtained solid is transferred to a Pyrex reaction bottle and placed in a microwave In the reactor, take it out after 15 minutes of microwave under 300W power. After the reaction is finished, the product is extracted with acetone, and then the acetone is removed to obtain a crude product, which is finally recrystallized with a mixed solvent of acetone and ethanol. The title compound (0.90 g) was obtained with a yield of 81.1%.
[0042] Yel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com