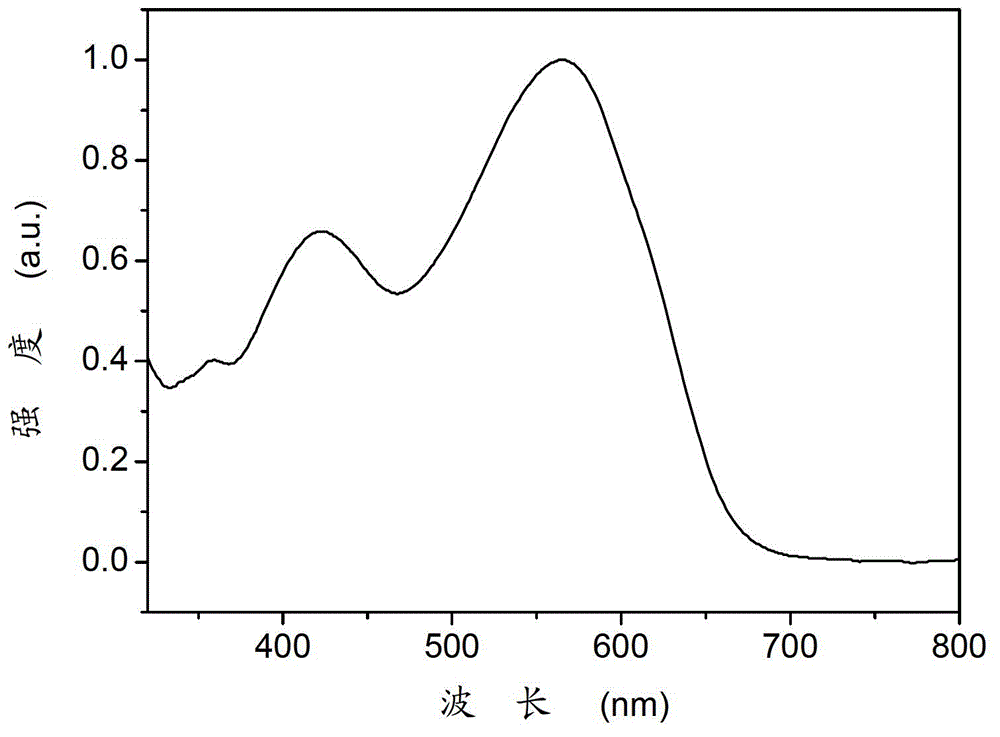
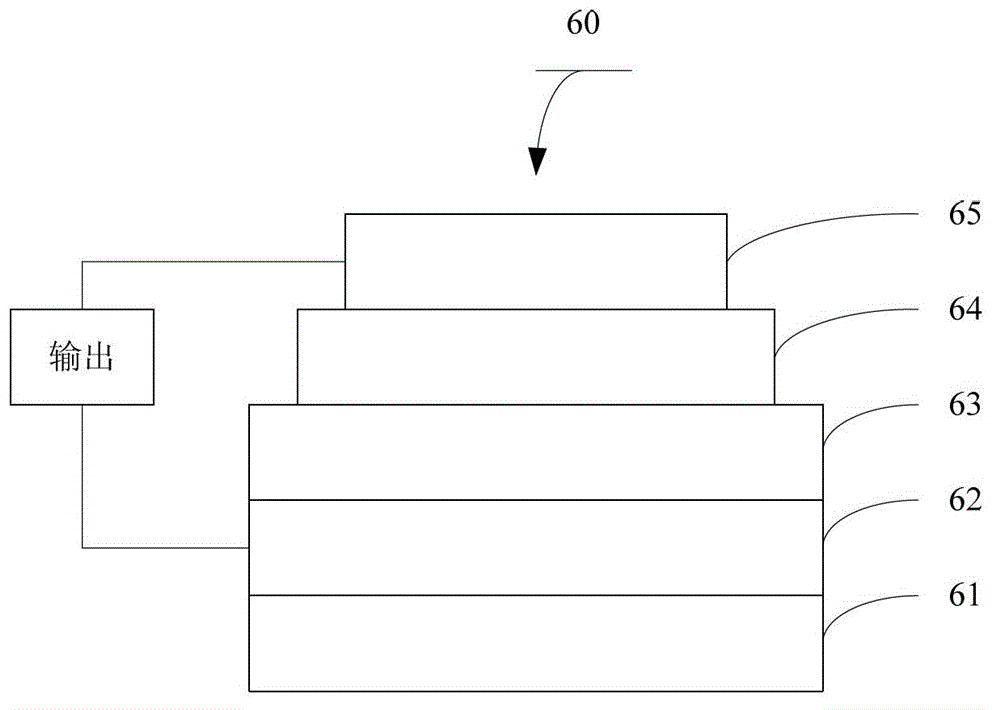
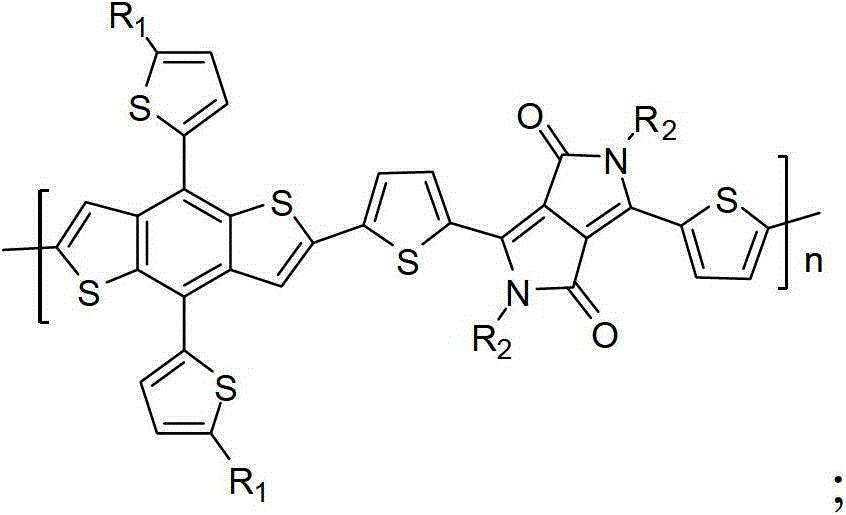
Polymer containing diketopyrrolopyrrole group as well as preparation method and application thereof
A rolopyrrole diketone-based polymer technology, which is applied in the field of organic solar cell materials, can solve the problems of low conversion efficiency of inorganic solar cells, low carrier electrode collection efficiency, and mismatched spectral response to achieve excellent photovoltaic performance , novel structure, and the effect of solving low efficiency problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The polymer containing diketopyrrolopyrrole in this example, namely poly{2,6-diyl-4,8-bis(5-n-octylthiophene)benzodithiophene-co-3,6- Bis(5-yl-2-thienyl)-2,5-dihydro-2,5-di-n-octylpyrrolo[3,4-c]pyrrole-1,4-dione} (P1), ( Among them, R1 is n-octyl, R2 is n-octyl, n=60), and its structural formula is as follows:
[0028]
[0029] The preparation steps of above-mentioned polymer are as follows:
[0030] The reaction formula is as follows:
[0031]
[0032] Under the protection of argon, 2,6-bistrimethyltin-4,8-bis(5-n-octylthiophene)benzodithiophene (181mg, 0.2mmol), 3,6-bis(5- Bromo-2-thienyl)-2,5-dihydro-2,5-di-n-octylpyrrolo[3,4-c]pyrrole-1,4-dione (136.4mg, 0.2mmol) was added into a In a flask containing 10ml of toluene solvent, deoxygenate in vacuum and fill with argon, then add bistriphenylphosphinepalladium dichloride (5.6mg, 0.008mmol); heat the flask to 100°C for Stille coupling reaction for 36h. Subsequently, the polymerization reaction was stopped afte...
Embodiment 2
[0036] The polymer containing the diketopyrrolopyrrole group in this example, that is, poly{2,6-diyl-4,8-bis(5-methylthiophene)benzodithiophene-co-3,6-bis( 5-yl-2-thienyl)-2,5-dihydro-2,5-dieicosylpyrrolo[3,4-c]pyrrole-1,4-dione} (P2), ( Among them, R1 is methyl, R2 is n-eicosyl, n=10), and its structural formula is as follows:
[0037]
[0038] The preparation steps of above-mentioned polymer are as follows:
[0039] The reaction formula is as follows:
[0040]
[0041] Under the protection of mixed gas of nitrogen and argon, 2,6-ditrimethyltin-4,8-bis(5-methylthiophene)benzodithiophene (212mg, 0.3mmol), 3,6-bis(5 -Bromo-2-thienyl)-2,5-dihydro-2,5-di-eicosylpyrrolo[3,4-c]pyrrole-1,4-dione (306mg, 0.3mmol) and Add 15mL of tetrahydrofuran into a 50mL two-necked bottle, fully dissolve it, pass in a mixture of nitrogen and argon to exhaust the air for about 20 minutes, then add tetrakistriphenylphosphine palladium (4mg, 0.003mmol) into it, and then fully pass through nit...
Embodiment 3
[0044] The polymer containing diketopyrrolopyrrole groups in this example, that is, poly{2,6-diyl-4,8-bis(5-n-eicosylthiophene)benzodithiophene-co-3,6 -bis(5-yl-2-thienyl)-2,5-dihydro-2,5-dimethylpyrrolo[3,4-c]pyrrole-1,4-dione} (P3), ( Among them, R1 is n-eicosyl, R2 is methyl, n=84), and its structural formula is as follows:
[0045]
[0046] The preparation steps of above-mentioned polymer are as follows:
[0047] The reaction formula is as follows:
[0048]
[0049] Under nitrogen protection, 2,6-ditrimethyltin-4,8-bis(5-n-eicosylthiophene)benzodithiophene (372mg, 0.3mmol), 3,6-bis(5-bromo -2-thienyl)-2,5-dihydro-2,5-dimethylpyrrolo[3,4-c]pyrrole-1,4-dione (160.4mg, 0.33mmol), palladium acetate (3.5 mg, 0.015mmol) and tri(o-methoxyphenyl)phosphine (21mg, 0.06mmol) were added into a flask containing 12mL of N,N-dimethylformamide, and then the flask was purged with nitrogen for about After 20 min; the flask was heated to 130° C. for Stille coupling reaction for 6 h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com