Preparation method of methylene succinyl chloride
A technology for the acylation of methylene succinyl and chloride, which is applied in the field of preparation of methylene succinyl chloride, can solve the problems of difficult control of carboxyl reaction, difficult temperature control, poor double bond activity, etc., and achieve stable quality, The effect of controllable reaction conditions and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The preparation method of methylene succinoyl chloride provided by the present invention comprises the steps of chloroacylation reaction of itaconic acid and chloroacylating reagent, crude product filtration and the like. The chloroacylation reaction of the present invention is carried out at an absolute pressure of 0-100Kpa and 30-200°C, and nitrogen gas is introduced into the system to facilitate the discharge of gas products. During the reaction, a solvent may or may not be used, and each reactant is reacted in a molten state.
[0020] The chloroacylating agent used in the present invention can be any one or more of thionyl chloride, phosphorus pentachloride, phosphorus trichloride, and benzoyl chloride.
Embodiment 1
[0022] The preparation method of the methylene succinoyl chloride described in the present embodiment specifically comprises the following steps:
[0023] Add 30 g of itaconic acid, 80 g of phosphorus pentachloride and 2 g of N,N-dimethylformamide into a 500 ml four-neck flask equipped with a stirrer, thermometer, exhaust gas outlet, and air inlet. The tail gas absorption system is an absorption bottle filled with 150ml 30% sodium hydroxide solution and a vacuum pump. Turn on the vacuum pump so that the gas in the four-neck flask is discharged through the absorption bottle, and the vacuum degree of the system is maintained at about 20KPa. The temperature was raised to 80°C, and the reaction was kept for 2 hours. After the reaction, the material was cooled to 20°C and filtered through a sand core funnel to obtain 32.3 g of methylene succinoyl chloride with a yield of 83.9%.
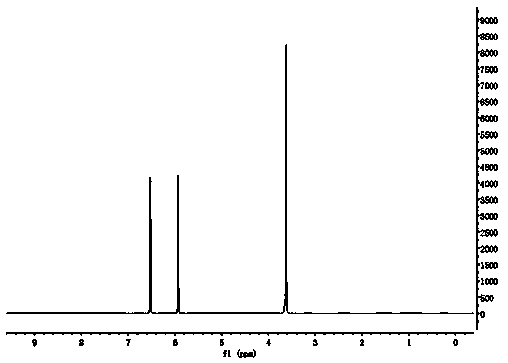
[0024] H NMR spectroscopy (such as figure 1 (shown) shows that: the chemical shifts at 6.52 and 5.93...
Embodiment 2
[0026] The preparation method of the methylene succinoyl chloride described in the present embodiment specifically comprises the following steps:
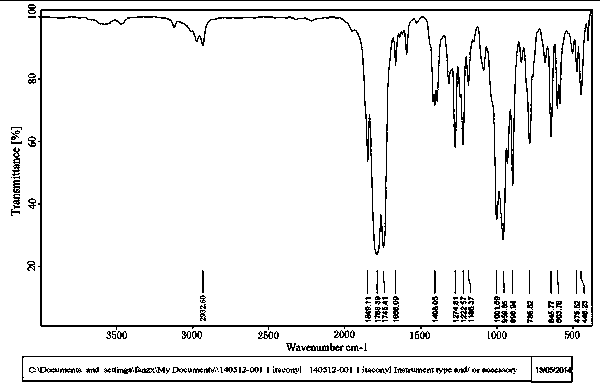
[0027] Add 30 g of itaconic acid, 32 g of thionyl chloride and 2 g of N,N-dimethylformamide into a 500ml four-neck flask equipped with a stirrer, thermometer, exhaust gas outlet, and air inlet, and turn on the vacuum pump to make The gas in the four-neck flask is discharged through the absorption bottle, and the system maintains a vacuum of 20KPa. Raise the temperature to 80°C, add 40 g of thionyl chloride to the constant-pressure dropping funnel, start to add the thionyl chloride dropwise, and keep the reaction for 2 hours. The temperature was raised to 110°C, and unreacted raw materials were evaporated under a negative pressure of 80°C. Cool to room temperature and filter with a sand core funnel to obtain 33.9 g of methylene succinoyl chloride with a yield of 88.1%. H NMR and IR spectrogram data are similar to Example 1, indica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com