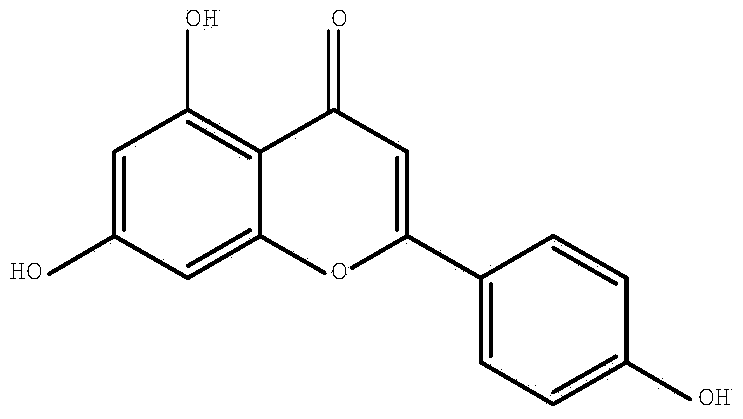
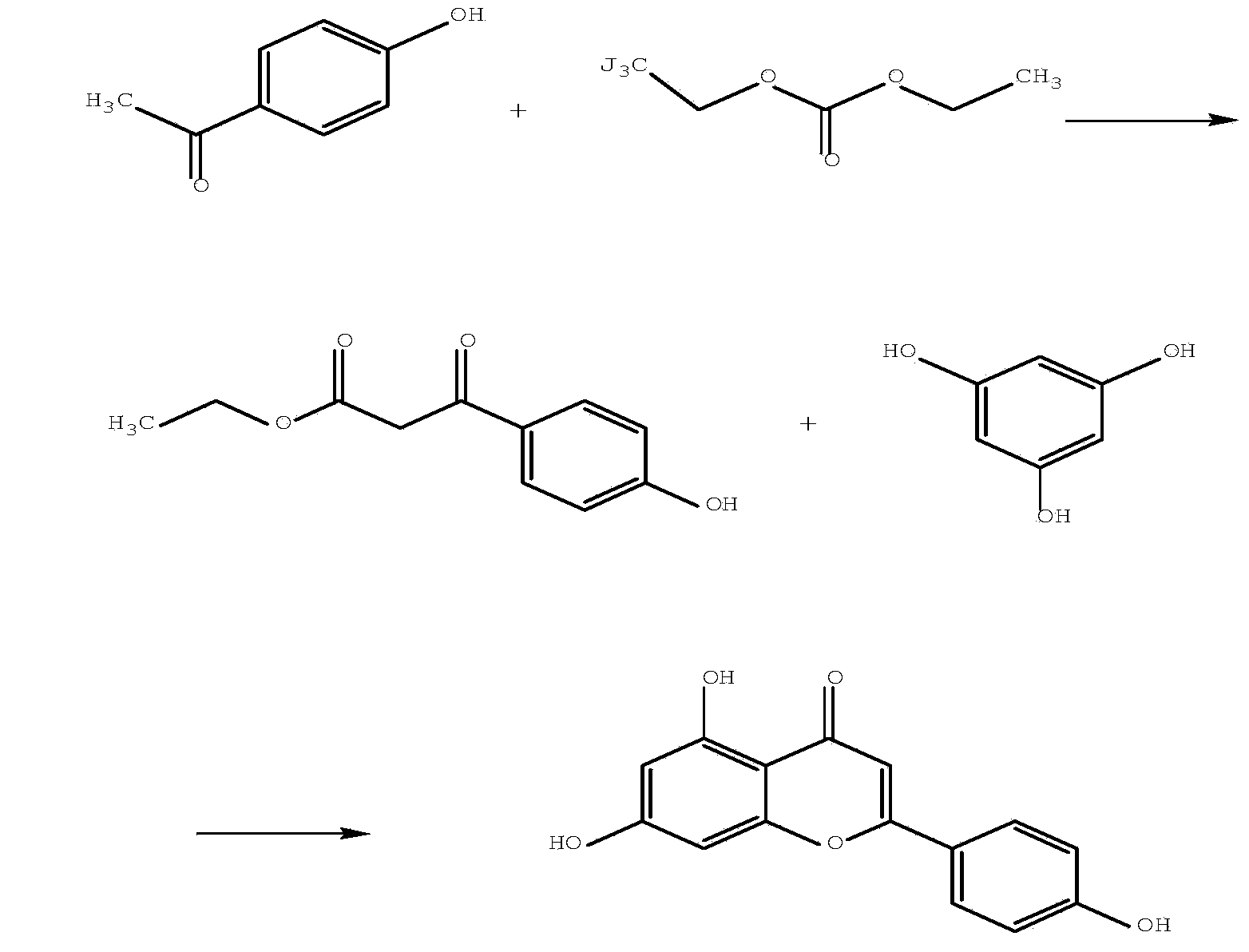
Synthetic method of apigenin
A synthesis method and technology of apigenin, applied in the direction of organic chemistry and the like, can solve the problems of unsuitable large-scale industrial production, harsh production conditions and high cost of apigenin, and achieve the effects of stable reaction, high equipment utilization rate and low production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The synthetic method of this apigenin comprises following synthetic route and step:
[0023]
[0024] Concrete technical scheme of the present invention is as follows:
[0025] 1] Condensation reaction (i.e. preparation of ethyl p-hydroxyphenylacetoacetate)
[0026] The key intermediate ethyl p-hydroxyphenylacetoacetate was prepared by condensation of p-hydroxyacetophenone and diethyl carbonate under alkaline conditions. Wherein the basic catalyst refers to strong basic catalysts such as sodium, sodium alkoxide, sodium hydride, sodium tert-butoxide, potassium tert-butoxide; preferred catalyst is sodium alkoxide; reaction solvent is benzene, toluene, ether, tetrahydrofuran, dehydrated alcohol, without water methanol etc. Preferred solvents are toluene, absolute ethanol or absolute methanol.
[0027] The specific operation process is:
[0028] Feed in the reaction bottle, p-hydroxyacetophenone, reaction solvent and basic catalyst, add diethyl carbonate solution dro...
example 1
[0035] Example 1 Feed into the reaction flask, 68g of p-hydroxyacetophenone, 160ml of anhydrous methanol, stir to dissolve, add 9g of sodium methoxide, after stirring evenly, slowly add 36g of diethyl carbonate dropwise at room temperature, about 2h to complete the dropwise addition. After dripping, the temperature was raised to reflux, and after about 7 hours, the reaction was stopped. The solvent was recovered under reduced pressure until nearly dry. Cool down to room temperature, add water, stir evenly, separate liquids, and dry over anhydrous sodium sulfate to obtain 59.5 g of the product (yield 82%).
example 2
[0036] Example 2 Feed into the reaction flask, diethyl carbonate 34g, anhydrous methanol 80ml, stir evenly, add sodium methoxide 12g, at room temperature, add dropwise (p-hydroxyacetophenone 68g+80ml) solution, drop about 3h. After the addition, the temperature was raised to reflux, and after about 8 hours, the reaction was stopped. The solvent was recovered under reduced pressure until nearly dry. Cool down to room temperature, add water, stir evenly, separate liquids, and dry over anhydrous sodium sulfate to obtain 56 g of the product (yield 78%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com