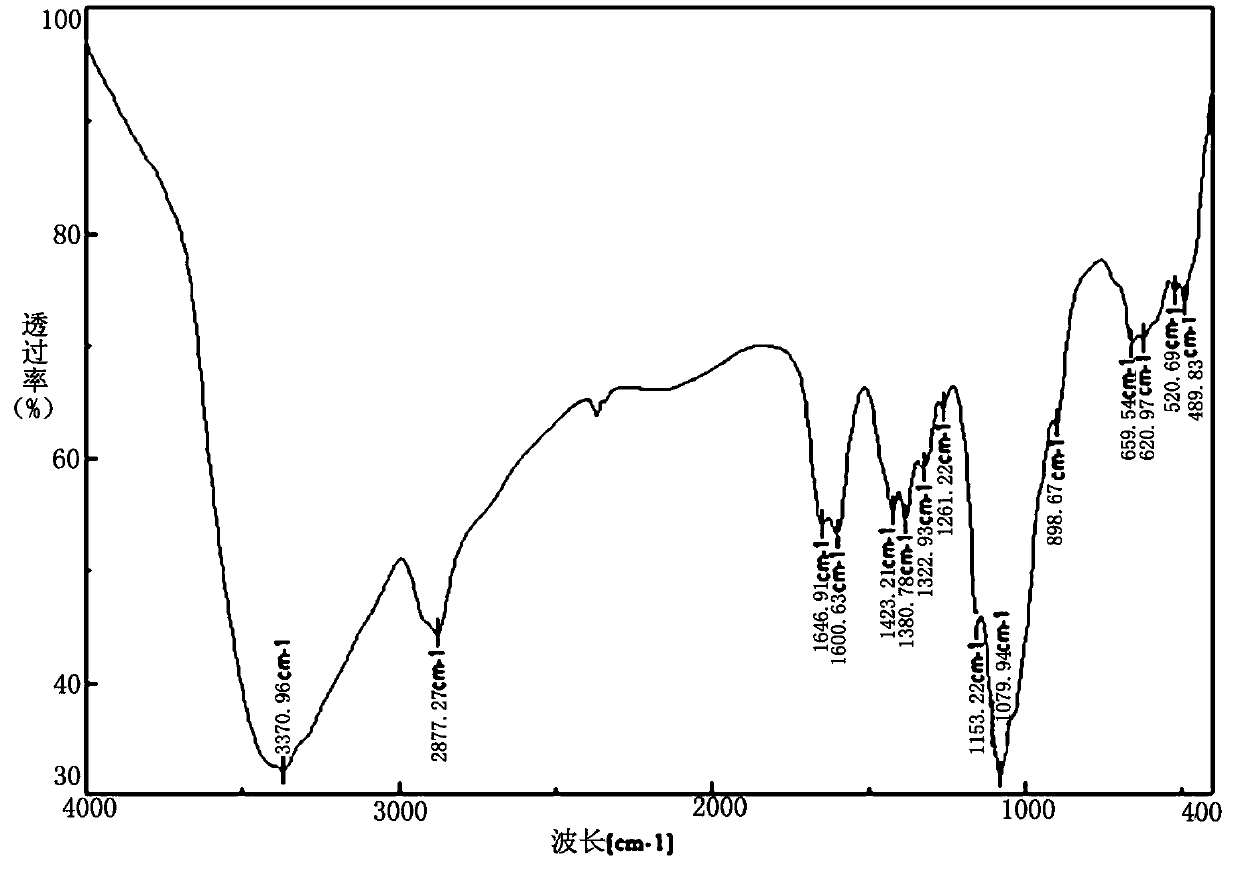
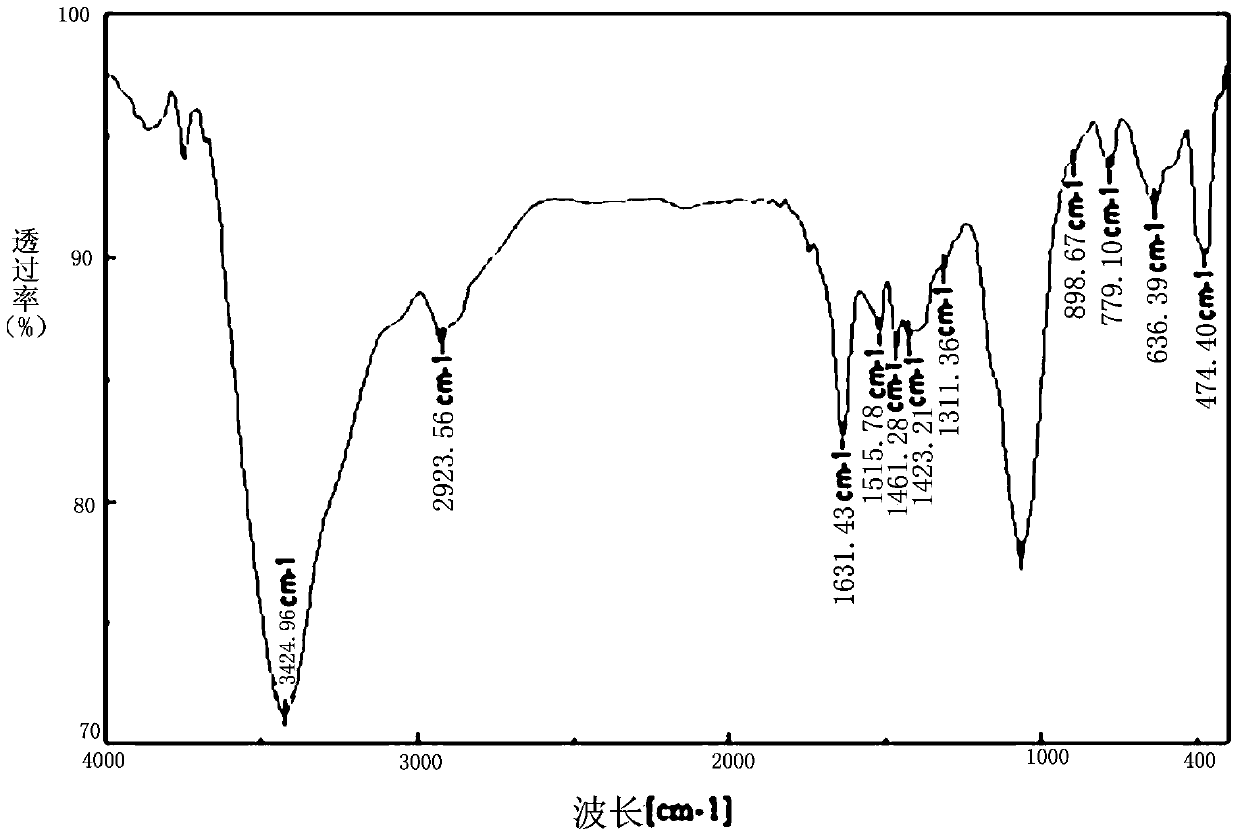
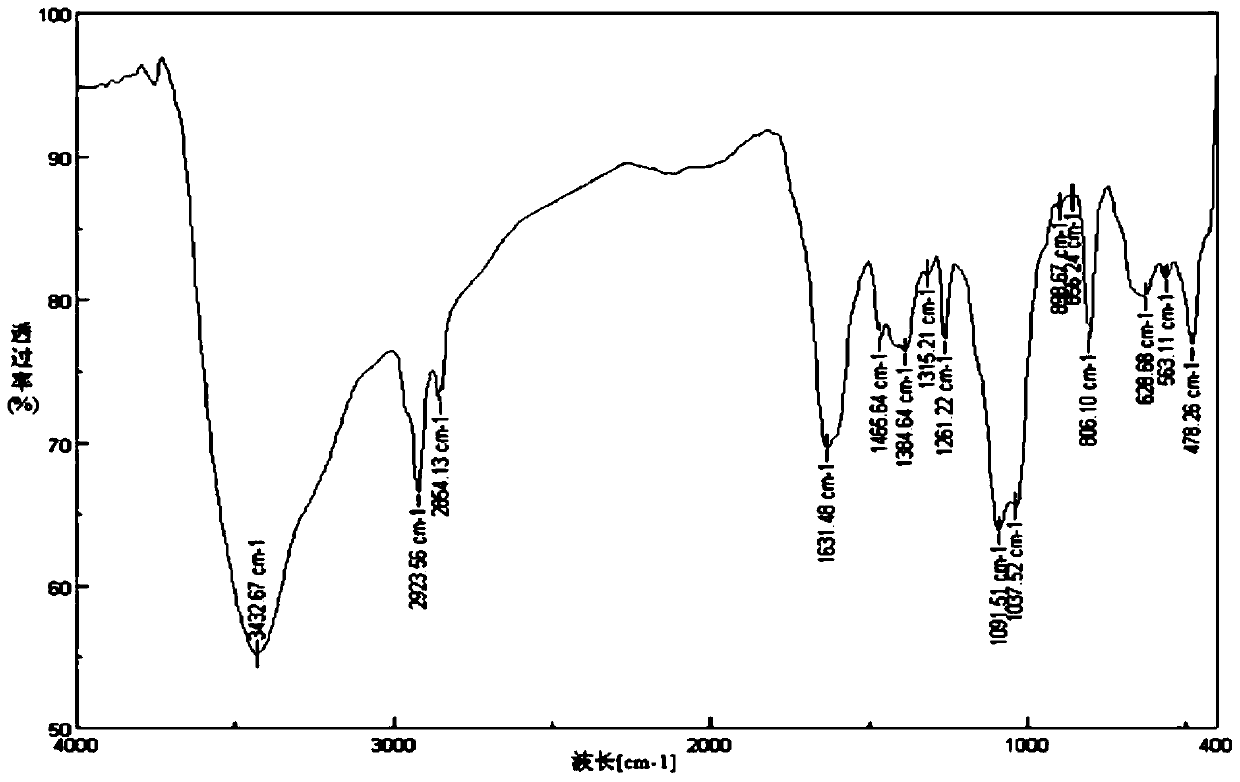
A kind of chitosan bispyridine quaternary ammonium salt and its preparation method and application
A technology of double quaternary ammonium salt and chitosan, applied in the field of daily chemicals, can solve the problems of affecting the use range of chitosan, poor water solubility, etc., and achieve the effects of good antioxidant activity, low cost, and weakening of hydrogen bonds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Chitosan diquaternary ammonium salt is a compound shown in formula 1, formula 2 and formula 3.
[0021] Preparation of chitosan diquaternary ammonium salt: 1.61g chitosan was dissolved in 50mL volume ratio of 2% acetic acid solution, after dissolving chitosan was added to 30mmol2-pyridineformaldehyde and 3-pyridine dissolved in 10mL absolute ethanol respectively Formaldehyde or 4-pyridine formaldehyde, continue to stir at room temperature for 2h, after stirring, add 45mmol sodium borohydride dropwise at a rate of 2mL per minute, stir for 2h, pour into excess acetone after stirring, precipitate, suction filter, dry at 60°C, grind , to obtain solid powders of N-substituted chitosan derivatives, respectively. Take 0.5g of the solid powder obtained in the previous step and disperse them in 30mL of N-methyl-2-pyrrolidone, stir at room temperature for 12h, add 0.1mL of 1M sodium hydroxide solution, 0.75g of sodium iodide and 4mL of methyl iodide, stir at 50°C for 20h, and rea...
Embodiment 2
[0024] The difference from Example 1 is:
[0025] Preparation of chitosan diquaternary ammonium derivatives: 1.61g chitosan was dissolved in 50mL volume ratio of 2% acetic acid solution. Or in 4-pyridine formaldehyde, continue to stir at room temperature for 1.5h, after stirring, add 45mmol sodium borohydride dropwise at a rate of 2mL per minute, stir for 2h, pour into excess acetone after stirring, precipitate, filter with suction, and dry at 60°C. Grinding to obtain solid powders of N-substituted chitosan derivatives respectively. Take 0.5 g of the solid powder obtained in the previous step and disperse them in 30 mL of N-methyl-2-pyrrolidone, stir at room temperature for 12 h, add 0.1 mL of 1M sodium hydroxide solution, 0.75 g of sodium iodide, and 4 mL of methyl iodide, stir at 50 ° C for 20 h, and react Pour into excess acetone, precipitate, filter with suction, wash with acetone for 3 times, and dry at 60°C to obtain the chitosan diquaternary ammonium salt derivative sh...
Embodiment 3
[0027] 1.61g chitosan was dissolved in 50mL volume ratio of 2% acetic acid solution, after dissolving chitosan was added to 30mmol 2-pyridine formaldehyde, 3-pyridine formaldehyde or 4-pyridine formaldehyde dissolved in absolute ethanol respectively, and stirring was continued at room temperature 2h, after stirring, add 45mmol sodium borohydride dropwise at a rate of 2mL per minute, stir for 2h, pour into excess acetone after stirring, precipitate, filter with suction, dry at 60°C, and grind to obtain N-substituted chitosan derivatives respectively of solid powder. Disperse 0.5 g of the solid powder obtained in the previous step into 30 mL of N-methyl-2-pyrrolidone, stir at room temperature for 6 h, add 0.1 mL of 1M sodium hydroxide solution, 0.75 g of sodium iodide, and 4 mL of methyl iodide, stir at 50 °C for 20 h, and react Pour into excess acetone, precipitate, filter with suction, wash with acetone for 3 times, and dry at 60°C to obtain the chitosan diquaternary ammonium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com