Method utilizing waste sulfuric acid and ferrous sulfate to produce polymeric ferric sulfate through oxygen pressurizing
A technology for polymerizing ferric sulfate and ferrous sulfate, applied in the direction of ferric sulfate, can solve the problems of consuming mineral resources and sulfuric acid resources, uneconomical, and environmentally friendly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
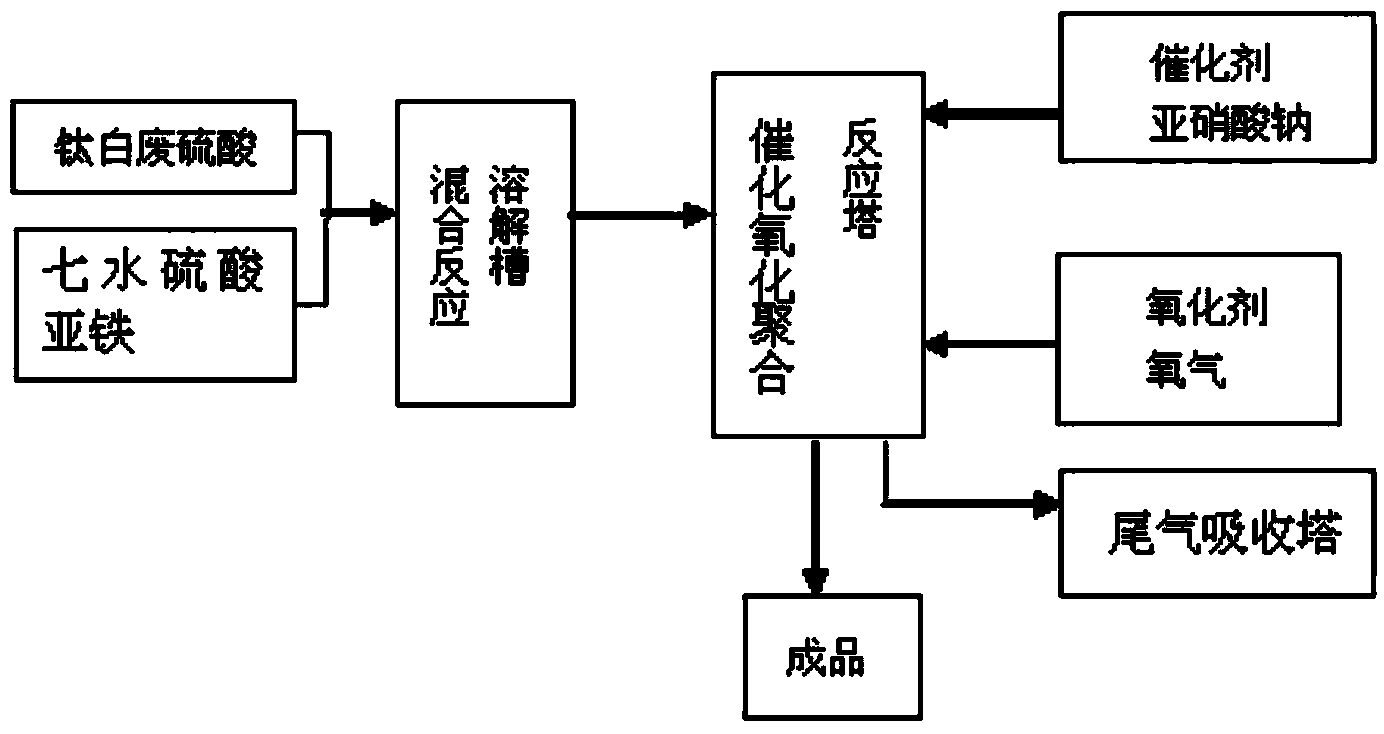
[0028] Utilize waste sulfuric acid and ferrous sulfate oxygen pressurization to produce the method for polyferric sulfate, comprise the following steps:
[0029] ⑴Acid mixed and dissolved
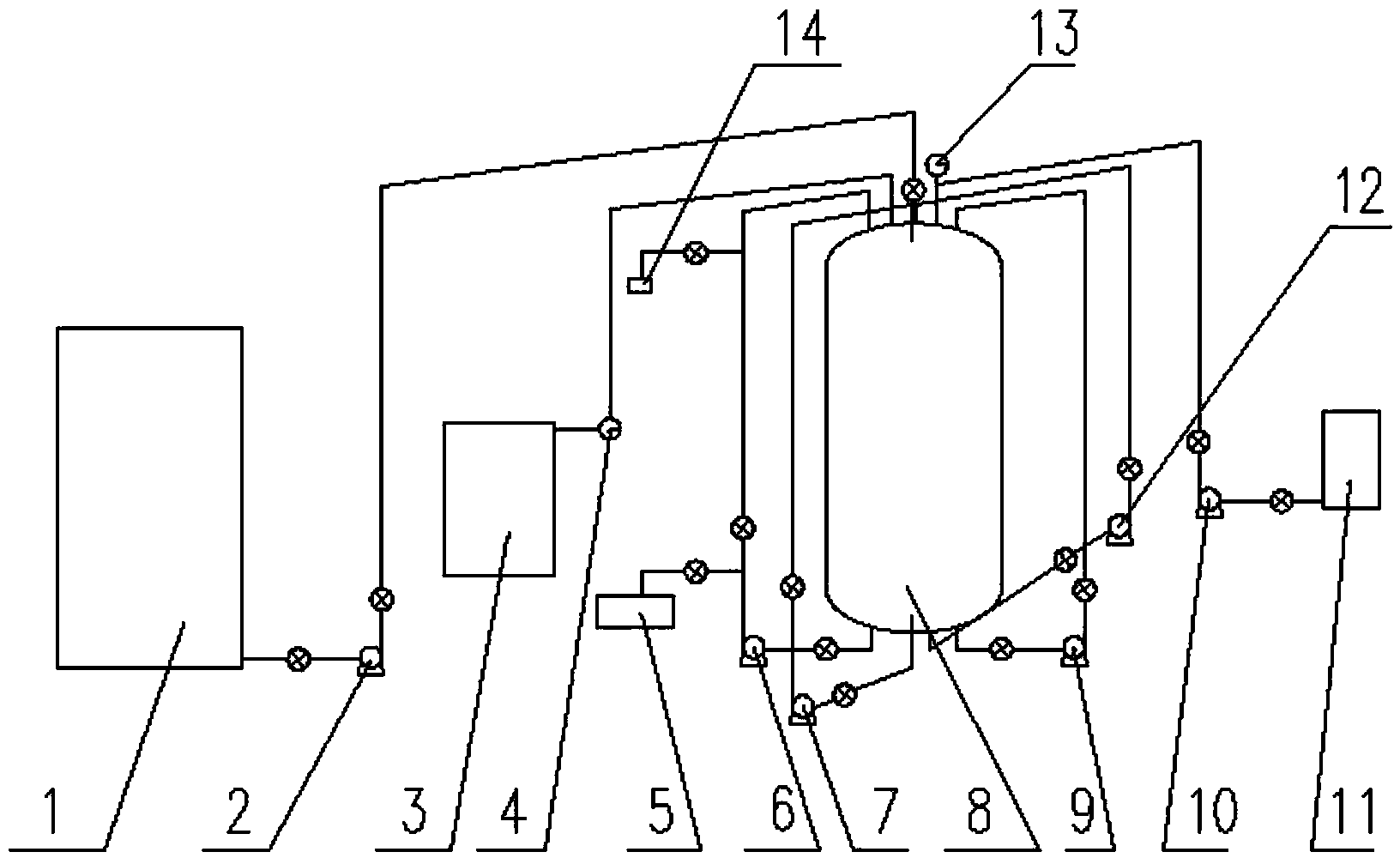
[0030] Extract 9 tons of waste sulfuric acid containing 3% iron and 20% sulfuric acid from the acid storage tank with a material pump 2, add it to the dissolving tank 1 with a stirrer, the stirring speed is controlled at 45r / min, and add 13 tons of waste sulfuric acid containing The ferrous sulfate heptahydrate with 18% iron has a mixing and dissolving temperature of 70° C. and a mixing and dissolving time of 20 minutes. After the ferrous sulfate heptahydrate is completely mixed and dissolved in the waste sulfuric acid solution, the end point of the reaction is achieved by stirring evenly.
[0031] ⑵ Catalytic Oxidative Polymerization
[0032] After the acid mixed dissolution reaction reaches the end point, keep the stirring speed at 45r / min, and use the suction pump 2 to pump the mixed an...
Embodiment 2
[0036] A kind of method utilizing waste sulfuric acid and ferrous sulfate oxygen pressure to produce polyferric sulfate, comprises the following steps:
[0037] ⑴Acid mixed and dissolved
[0038] Extract 9 tons of iron-containing 3%; sulfuric acid 20% iron waste sulfuric acid from the acid storage tank with the pump 2, add in the dissolving tank 1 with a stirrer, control the stirring speed at 44r / min, add 13 tons while stirring Ferrous sulfate heptahydrate containing 18% iron, after ferrous sulfate heptahydrate is completely mixed and dissolved in the waste sulfuric acid solution, the acid dissolution temperature is 50°C, the time is 25 minutes, and the reaction end point is achieved by stirring evenly.
[0039] ⑵ Catalytic Oxidative Polymerization
[0040] After the acid mixed dissolution reaction reaches the end point, keep the stirring speed at 44r / min, use the pump 2 to pump the mixed and dissolved material in the dissolution tank 1 to the circulation reaction tower 8, ad...
Embodiment 3
[0044] A kind of method utilizing waste sulfuric acid and ferrous sulfate oxygen pressure to produce polyferric sulfate, comprises the following steps:
[0045] ⑴Acid mixed and dissolved
[0046] Extract 9 tons of iron-containing 3%; sulfuric acid 20% iron waste sulfuric acid from the acid storage tank with the material pump 2, add in the dissolution tank 1 with a stirrer, control the stirring speed at 46r / min, add 13 tons while stirring The ferrous sulfate heptahydrate containing 18% iron is completely mixed and dissolved in the waste sulfuric acid solution, the mixing and dissolving temperature is 30°C, the mixing and dissolving time is 30 minutes, and the reaction end point is achieved by stirring evenly.
[0047] ⑵ Catalytic Oxidative Polymerization
[0048] After the acid mixed dissolution reaction reaches the end point, keep the stirring speed controlled at 46r / min, use the pump 2 to pump the mixed and dissolved material in the dissolution tank to the circulation reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com