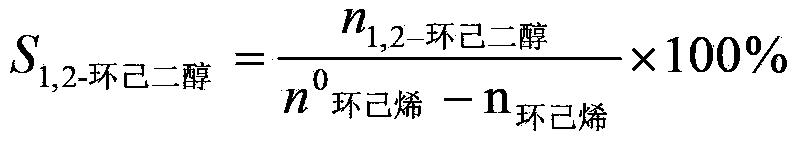Preparation method of o-dihydroxybenzene
A technology of catechol and cyclohexanediol, which is applied in the field of preparing catechol, can solve the problems of high energy consumption, prolong catalyst life, and difficulty, and achieve the effects of reduced energy consumption, high yield, and prolonged life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
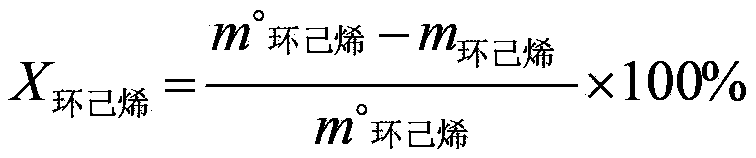
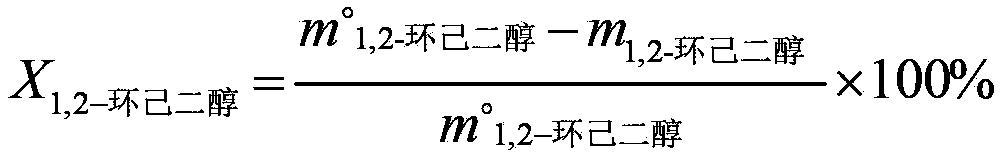
[0041] (1) Using acetone as an organic solvent, contact cyclohexene and hydrogen peroxide (30% by mass) with titanium-silicon molecular sieve TS-1, wherein the molar ratio of cyclohexene to hydrogen peroxide calculated as hydrogen peroxide is 1:1.2, The mass ratio of acetone, titanium silicon molecular sieve and cyclohexene is 15:0.05:1, and hydrochloric acid is added to adjust the pH value to 4, and they are contacted at 60°C for 3 hours to obtain a reaction solution containing 1,2-cyclohexanediol (cyclohexanediol The conversion rate of hexene is 98.1%, the selectivity of 1,2-cyclohexanediol is 98.8%), the reaction liquid containing 1,2-cyclohexanediol is successively passed through cation exchange resin and anion exchange resin to remove the Anions (such as chloride ions, etc.) and cations (such as titanium ions, etc.) to obtain a dehydrogenation stock solution;
[0042] (2) Put the dehydrogenation catalyst into the reaction kettle, then introduce the dehydrogenation stock s...
Embodiment 2
[0044] Catechol was prepared according to the method of Example 1, except that the liquid-phase catalytic dehydrogenation conditions in step (2) were changed, and the results are shown in Table 1.
[0045] Table 1
[0046]
[0047]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com