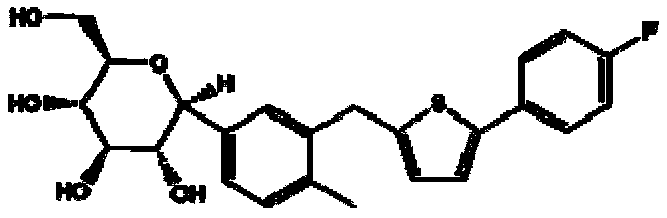
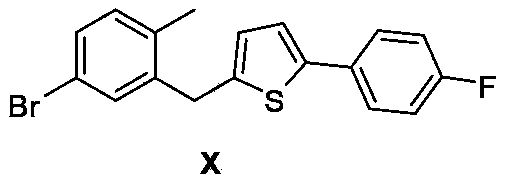
5-(5-bromo-2methylphenyl)-1-(4-fluorophenyl)pentane-1,4-dione, preparation method and applications thereof
A technology of methylphenyl and fluorophenyl, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, and can solve problems such as unsuitable for industrialization, cumbersome steps, and complicated preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
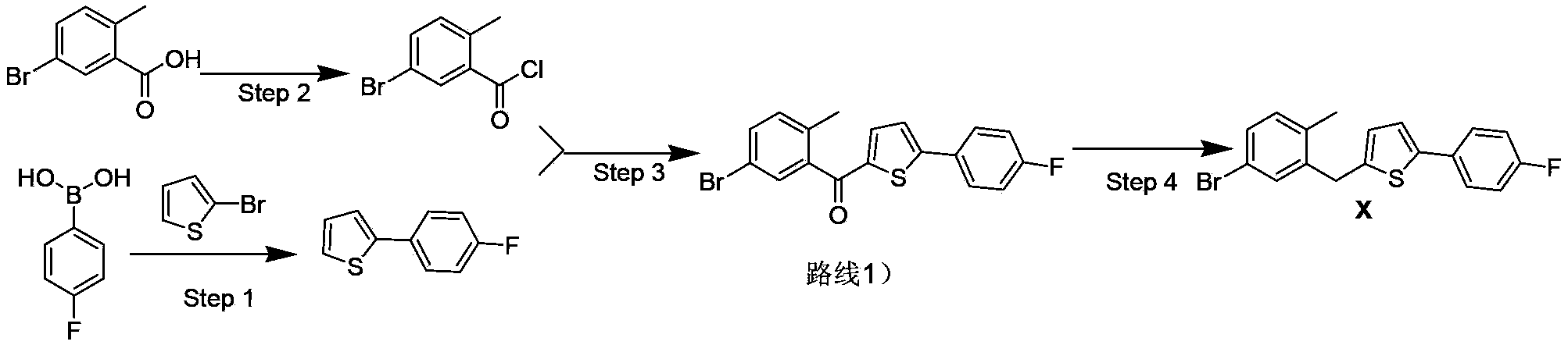
[0029] Synthesis of Example 13-(5-bromo-2-methylphenyl)-2-oxopropionic acid (formula III)
[0030] The synthesis of 3-(5-bromo-2-methylphenyl)-2-oxopropionic acid refers to the literature of Raap et al. (Eur.J.Org.Chem.1999, 2609-2621).
[0031] Add 2-methyl-5-bromobenzaldehyde (20g, 100mmol), hydantoin (15g, 150mmol), sodium acetate (20g, 244mmol) and 150mL glacial acetic acid into a 250mL reaction flask, heat the reaction system to reflux overnight, and cool the system to room temperature, slowly pour into 500mL ice-water system, lower to room temperature, filter, and wash the filter cake with 500mL water until neutral. Collect the solid, add the solid into a 250mL reaction flask, add 100mL of 20% NaOH solution, reflux the system for 3h under the protection of nitrogen, cool down to 20-30°C, and neutralize the system with 6N hydrochloric acid aqueous solution to pH=7-8, 10 g of solid sodium bicarbonate was added, and the system was extracted three times with ethyl acetate. ...
example 25
[0033] Synthesis of Example 25-(5-bromo-2-methylphenyl)-1-(4-fluorophenyl)pentane-1,4-diketone (Formula I)
[0034] Method 1): In a 500mL three-necked flask, add 3-(5-bromo-2-methylphenyl)-2-carbonylpropionic acid (formula III, 25.7g, 100mmol), 1-(4-fluorophenyl)- 2-ene-1-propanone (formula II, 15g, 100mmol), triethylamine (10.1g, 100mmol), 5-(2-hydroxyethyl)-4-methyl-3-benzyl-thiazole chloride (2.7g, 10mmol), ethanol 150mL, heat the system to reflux, cool down after 15h, add 100mL of 10% saline to the system, extract with 200mL ethyl acetate, concentrate the organic phase, and perform column chromatography (PE / EA=7 / 1) 27.6 g of the compound of formula I was obtained, with a yield of 76%.
[0035] MS(ESI)363.0(M+H + , 100%)
[0036] Method 2): In a 500mL three-necked flask, add 3-(5-bromo-2-methylphenyl)-2-carbonylpropionic acid (formula III, 25.7g, 100mmol), 1-(4-fluorophenyl)- 2-ene-1-propanone (formula II, 15g, 100mmol), triethylamine (10.1g, 100mmol), 5-(2-hydroxyethy...
example 3
[0042] Synthesis of Example 32-(5-bromo-2-methylbenzyl)-5-(4-fluorophenyl)thiophene (formula X)
[0043] Method 1): Add 5-(5-bromo-2-methylphenyl)-1-(4-fluorophenyl)pentane-1,4-dione (Formula I, 18g, 50mmol) into a 250mL three-necked flask , toluene 100mL, Lowe's reagent (20g, 50mmol), the system was refluxed for 6h, TLC detected that all the raw materials I disappeared, the system was cooled down, concentrated and then column chromatographed (PE / EA=3 / 1) to obtain 14g of the compound of formula X. The rate is 78%.
[0044] MS(ESI)361.0(M+H + , 100%)
[0045] Method 2): Add 5-(5-bromo-2-methylphenyl)-1-(4-fluorophenyl)pentane-1,4-dione (Formula I, 18g, 50mmol) into a 250mL three-necked flask , xylene 100mL, Lowe’s reagent (20g, 50mmol), the system was refluxed for 8h, TLC detected that all the raw materials I disappeared, the system was cooled down, concentrated and then column chromatographed (PE / EA=3 / 1) to obtain 15g of the compound of formula X, The yield is 83%.
[004...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com